
Funda Meric-Bernstam, MD, highlights considerations with pan-tumor approvals and where testing and reporting should be improved for clarity/ease.

Your AI-Trained Oncology Knowledge Connection!


Megan Hollasch joined MJH Life Sciences in 2022 following graduation from the University of Massachusetts Amherst with majors in Communication and English, and a specialization in Literature as History. She produces and edits original content for the print publication OncologyLive, aiding in all aspects of the publication process, as well as content for OncLive.com.

Funda Meric-Bernstam, MD, highlights considerations with pan-tumor approvals and where testing and reporting should be improved for clarity/ease.

Rohan Maniar, MD, details considerations when treating patients with rare thymic epithelial tumors as well as notable ongoing research in the field.

Following its approval in high-risk BCG-unresponsive NMIBC with CIS, nadofaragene firadenovec is being assessed in intermediate-risk disease in ABLE-32.

Amy M. Ahnert, MD, highlights newer shared risk factors for CVD and breast cancer and questions regarding personalized screening for the diseases.

Amy M. Ahnert, MD, details the significance of findings on the link between CVD and breast cancer as well as next steps to further define the connection.
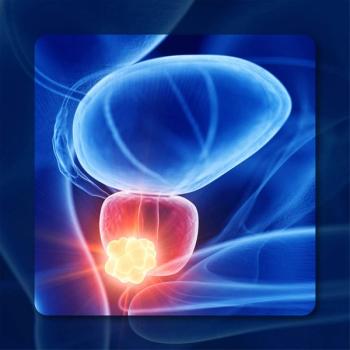
Adherence rates improved the longer patients with metastatic and nonmetastatic prostate cancer received relugolix for a high overall adherence rate.

Ritu Salani, MD, MBA, details the significance of trials examining avutometinib plus defactinib and enthusiasm for ADCs in ovarian cancer.
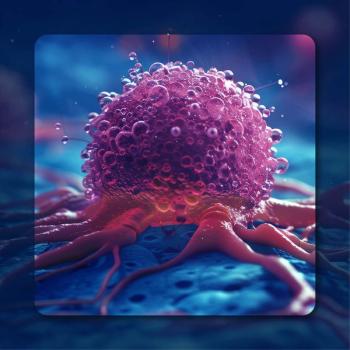
An ER model analysis showed that 1.34 mg of tivozanib provided a greater decrease in tumor size and may be more tolerable than an 0.89-mg dose in RCC.
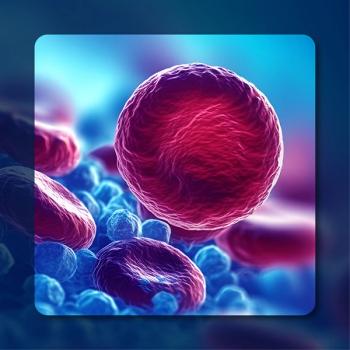
One-year follow-up data from an ongoing phase 1 study showed that Orca-Q offered similar benefits as T-cell depletion, without the typical compromises seen.

See this full list of regulatory decisions from 2024 regarding withdrawn agents, label updates, and new drug formulations.

Experts provide insights on the 2024 FDA approvals in several different fields including solid tumors and more in this list featuring the regulatory decisions.

Ritu Salani, MD, MBA, details considerations for cervical cancer treatment as new approvals, research, and modalities affect several settings.

This full list of 2024 FDA approvals in lung cancer features insights from experts who sat down with us throughout the year to discuss their significance.

The movement of CAR T-cell therapy to earlier lines of therapy was among many notable advancements greenlit by the FDA in the hematologic space in 2024.

Vikram M. Narayan, MD, details updates in the bladder cancer field, including effects of the BCG shortage and upcoming therapies/tools of interest.

Experts provide insights on the 2024 FDA approvals in the gastrointestinal and genitourinary fields in this list featuring all the regulatory decisions.

Kevin T. Nead, MD, MPhil, details findings on the association between prevalent CVD and breast cancer and next steps for investigating the connection.

Leveraging synthetic lethal approaches in lung cancer with new targets and agents may be a promising avenue of investigation.

Optimized T-cell manufacturing is one strategy that may prevent SPCs after CAR T-cell therapy, according to Shyam A. Patel, MD, PhD, and Saurabh Dahiya, MD, FACP.

Coauthor of the Lancet Breast Cancer Commission report, Reshma Jagsi, MD, DPhil, details the 6 themes of the evidence-based plan aimed to address challenges in the field.

Shyam A. Patel, MD, PhD, and Saurabh Dahiya, MD, FACP, detail the pathobiology of SPCs after CAR T-cell therapy and findings from a review on the cancers.

Findings from an oncogene overlap study in NSCLC support the potential clinical impact of high-level amplification of MET, HER2, and KRAS defined by NGS.

Alexander Watson, MD, DPhil, FRCPC, explains how the relationship between driver positivity and amplification status affects same-gene alteration enrichment.
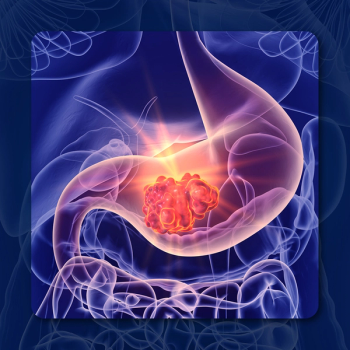
A subgroup analysis of the CABINET trial showed that cabozantinib extended PFS vs placebo in extrapancreatic NETs with a primary tumor arising in the GI tract.

Alexander Watson, MD, DPhil, FRCPC, details findings showing oncogene overlap is adequately applied to NGS-based tissue sampling by decreasing frequency and increasing copy number gain.

The CMS has selected 15 drugs covered under Medicare Part D for the second cycle of the Drug Price Negotiation Program, including enzalutamide, pomalidomide, palbociclib, and acalabrutinib.
E. Gabriela Chiorean, MD, FASCO, discusses exciting targeted therapies in the GI space and topics to be discussed at the School of Gastrointestinal Oncology meeting.

The 2025 American Cancer Society Annual Report Showed incidence rates are increasing for many cancer types and disparities remain regarding cancer mortality.
The second-line setting for HER2-mutated NSCLC is shifting with TKIs looking to demonstrate efficacy and T-DXd under examination in the frontline now.

Alison Schram, MD, details data from the eNRGy study that supported the FDA approval of zenocutuzumab in NRG1 fusion–positive NSCLC and pancreatic adenocarcinoma.