Hematologic Oncology
Latest News
Latest Videos

CME Content
More News

We had the pleasure of speaking with faculty from an OncLive® Institutional Perspectives in Cancer webinar on hematologic malignancies, hosted in partnership with Cleveland Clinic, to discuss current practice patterns and emerging therapies in chronic myeloid leukemia, chronic lymphocytic leukemia, myelofibrosis, and acute lymphoblastic leukemia.

Andrew M. Evens, DO, MSc, discusses the design of a retrospective analysis evaluating in relapsed/refractory classic Hodgkin lymphoma.
Dr Maziarz provides an overview of adverse events with CAR T-cell therapies and approaches to managing toxicities.
Panelists discuss data with CAR T-cell therapies in treatment of relapsed and refractory DLBCL.
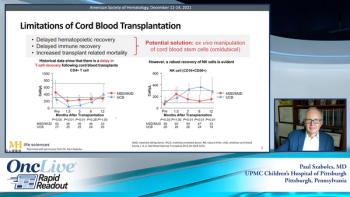
Expert hematologist/oncologist Paul Szabolcs, MD, reviews positive data from a clinical trial comparing hematopoietic stem cell transplantation and omidubicel with standard cord blood transplantation.
Drs Bussel, Piatek and Kessler discuss treatment of older patients with ITP.

Dr Bussel discusses the use of fostamatinib in patients who have progressed on TPO agents.

Treatment with rituximab prior to COVID-19 vaccination nearly halved the number of patients with B-cell non-Hodgkin lymphoma who developed blocking antibodies following COVID-19 vaccination compared with healthy controls.
Experts share their personal experience with asciminib as later-line therapy for patients with chronic myeloid leukemia.

Shared insight on asciminib’s role in chronic myeloid leukemia in light of available clinical data.

Tycel Phillips, MD, discusses a phase 1/2 trial combining subcutaneous epcoritamab with rituximab and lenalidomide in patients with relapsed or refractory follicular lymphoma, as presented at the 63rd ASH Annual Meeting in 2021.

Shared insight on updates in the chronic myeloid leukemia treatment landscape following the ASH 2021 annual meeting.

The FDA has announced that they are investigating umbralisib (Ukoniq), an oral inhibitor of PI3K-delta and CK1-epsilon that is approved to treat patients with marginal zone lymphoma and follicular lymphoma, after initial data from the phase 3 UNITY-CLL trial revealed a potential increased risk of death in those who received the agent.

Experts in hematology-oncology review the case of a 59-year-old woman with relapsed/refractory multiple myeloma and discuss their thoughts on the given treatment approach.
Sagar Lonial, MD; Joshua Richter, MD; and Ola Landgren, MD, PhD, comment on the selection of the appropriate treatment regimen at first relapse, including CD38-based, non-CD38–based and non-PI–based treatment regimens for multiple myeloma, and discuss their approach to assessing treatment response.

In 2021, 13 novel agents were approved across hematology/oncology. Additionally, there were many other notable approvals—whether they were new formulations, expanded indications, or biosimilar approvals—across tumor types that expanded accessibility for varying patient populations.

Expert perspectives on novel approaches to treatment in the relapsed/refractory setting, particularly CAR T-cell therapy, for patients with follicular lymphoma.

Shared insight on the indolent nature of follicular lymphoma and frontline therapy options for this disease.

Naveen Pemmaraju, MD, discusses results from a retrospective study examining the utilization hyper-CVAD, and long-term follow-up data regarding the use of tagraxofusp in patients with blastic plasmacytoid dendritic cell neoplasm.

The combination of brentuximab vedotin and chemotherapy resulted in a statistically significant improvement in overall survival when used in the frontline treatment of patients with advanced classical Hodgkin lymphoma.
Dr Miklos provides an overview of chimeric antigen receptor T (CAR-T) cell therapy.
Dr Nowakowski discusses patient heterogeneity and treatment of relapsed vs refractory patients with DLBCL.
The submission of a supplemental biologics license application seeking the approval of a Monday/Wednesday/Friday intramuscular dosing schedule for asparaginase erwinia chrysanthemi-rywn for use as a component of a multiagent chemotherapy regimen in patients with acute lymphoblastic leukemia and lymphoblastic lymphoma who have developed hypersensitivity to Escherichia coli–derived asparaginase has been submitted to the FDA.

ITP experts discuss use of splenectomy for treatment of ITP in their practices.

Physicians share concerns with ITP medications and response to COVID-19 and other vaccinations.