Melanoma & Skin Cancer
Latest News

Latest Videos

CME Content
More News

Predictive genetic signatures and novel combination strategies may be the key to improving the often dramatic responses seen with immune checkpoint inhibitors.

Sancy Leachman, MD,PhD, Professor and Chair, Department of Dermatology Director, Melanoma Research Program, Knight Cancer Institute, Oregon Health & Science University, discusses a new tool that oncologists can use with patients to identify melanoma recurrences.

Sheri Holmen, PhD, investigator, Associate Professor in the Department of Surgery, Adjunct Professor in the Department of Oncological Sciences, Huntsman Cancer Institute, the University of Utah School of Medicine, discusses understanding drivers for melanoma metastasis.

The FDA has granted a breakthrough therapy designation to avelumab as a potential treatment for patients with metastatic Merkel cell carcinoma following progression on at least one prior chemotherapy regimen.


The FDA has approved the combination of vemurafenib and cobimetinib as a treatment for patients with metastatic or unresectable BRAF V600E/K mutation-positive melanoma.

Howard L. Kaufman, MD, FACS, Chief Surgical Officer, Associate Director for Clinical Science, Surgical Oncologist, Rutgers Cancer Institute, Society for Immunotherapy of Cancer (SITC) President, discusses the impact of the recent FDA approval of talimogene laherparepvec (T-VEC) for the treatment of melanoma lesions in the skin and lymph nodes.

Adding the MEK inhibitor trametinib to the BRAF inhibitor dabrafenib significantly improves long-term outcomes while lowering certain adverse events associated with either agent alone for patients with BRAF-mutated metastatic melanoma.

Jeffrey S. Weber, MD, PhD, deputy director, Laura and Isaac Perlmutter Cancer Center, co-director of its Melanoma Program and Head of Experimental Therapeutics, NYU Langone Medical Center, discusses the significance of the FDA approval of the combination of dabrafenib and trametinib for patients with unresectable or metastatic melanoma who harbor a BRAF V600E or V600K mutation.

Combinations of targeted therapies continue to advance toward full regulatory approval for patients with metastatic or unresected melanoma, given the substantial benefits seen with these agents.

The FDA expanded the approval of ipilimumab (Yervoy) in melanoma to include adjuvant treatment of patients with stage III melanoma at high risk of recurrence following complete resection.

The FDA has approved talimogene laherparepvec for the treatment of patients with advanced melanoma.

Proinflammatory cytokines were elevated in patients with melanoma undergoing the combination treatment of radiation therapy and the systemic anti–CTLA-4 immunotherapy, ipilimumab.

Talimogene laherparepvec has received a positive recommendation from the CHMP, which suggests that the treatment should gain European approval for patients with advanced melanoma.
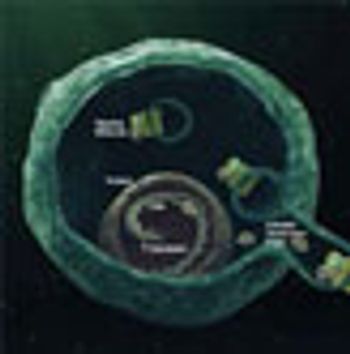
The Hedgehog pathway is one of several key developmental cell-signaling networks whose aberrant activation in adult tissues can lead to cancer.

Patricia M. LoRusso, DO, Professor of Medicine and Associate Director of Innovative Medicine, Yale Cancer Center, discusses taking a personalized medicine approach to treat melanoma patients whose tumors do not have BRAF alterations.


The combination of vemurafenib and cobimetinib demonstrated a statistically significant improvement in overall survival compared with vemurafenib alone for previously untreated patients with BRAFV600-mutant unresectable or metastatic melanoma.

The FDA has granted an accelerated approval to the combination of nivolumab and ipilimumab as a treatment for patients with BRAF V600 wild-type unresectable or metastatic melanoma.

The combination of dabrafenib plus trametinib improved overall survival by 7.6 months compared with single-agent vemurafenib in patients with unresectable or metastatic BRAFV600E/K-mutant melanoma.

The combination of the attenuated oncolytic virus talimogene laherparepvec and the immune checkpoint inhibitor pembrolizumab for unresectable melanoma has passed an early safety evaluation.

Two strategies of sequential immunotherapy for advanced melanoma showed similar safety and tolerability but substantially different efficacy.

The FDA has granted a priority review designation to a supplemental biologics license application for nivolumab plus ipilimumab in previously untreated patients with advanced melanoma.

Immunotherapy expert Jeffrey Weber, MD, PhD, will join NYU Langone Medical Center's Laura and Isaac Perlmutter Cancer Center as its deputy director and co-director of its melanoma program.