
Rivaroxaban may significantly reduce venous thromboembolism occurrence among patients actively being treated with systemic therapy, according to results from the phase IIIb CASSINI trial.

Your AI-Trained Oncology Knowledge Connection!


Rivaroxaban may significantly reduce venous thromboembolism occurrence among patients actively being treated with systemic therapy, according to results from the phase IIIb CASSINI trial.

Patients with relapsed/refractory chronic lymphoblastic leukemia treated with single-agent venetoclax attained high rates of minimal residual disease in peripheral blood and bone marrow.

The combination of ibrutinib and rituximab significantly improved overall survival and progression-free survival compared with standard fludarabine, cyclophosphamide, and rituximab for younger patients with chronic lymphocytic leukemia.

A Selinexor combination regimen induced an overall response rate of 26.2% in heavily pretreated patients with penta-refractory multiple myeloma.
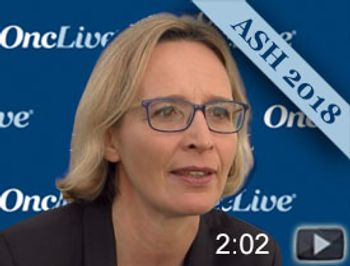
Viola Poeschel, MD, department of hematology, oncology and rheumatology, Saarland University Medical School, Germany, discusses outcomes of patients with diffuse large B-cell lymphoma (DLBCL) who were treated with 4 cycles of R-CHOP during the 2018 ASH Annual Meeting.

Nina Shah, MD, associate professor of medicine at the University of California, San Francisco Helen Diller Comprehensive Cancer Center, discusses initial results from a phase I clinical trial of bb21217, a next-generation anti-BCMA CAR T-cell therapy, in patients with multiple myeloma during the 2018 ASH Annual Meeting.

Axicabtagene ciloleucel elicited a 2-year overall survival rate of 51% in patients with refractory large B cell lymphoma, representing a clear plateau in the survival curve.

The overall survival benefit with quizartinib in patients with relapsed/refractory FLT3-ITD-mutated acute myeloid leukemia was observed across patient subgroups and reproduced consistently across sensitivity analyses.

The first-line combination of ibrutinib (Imbruvica) and obinutuzumab (Gazyva) was associated with a 77% reduction in the risk for disease progression or death compared with chemoimmunotherapy in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma.

Long-term follow-up of acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma shows that responses are durable with no new safety signals observed.

The R2 regimen of lenalidomide (Revlimid) plus rituximab (Rituxan) reduced the risk of disease progression or death by 54% versus rituximab alone in patients with relapsed/refractory indolent non-Hodgkin lymphoma.

The addition of a CD79b-targeted antibody-drug conjugate to bendamustine and rituximab more than doubled overall survival in patients with relapsed/refractory diffuse large B-cell lymphoma.

More than 70% of older patients ineligible for intensive chemotherapy for acute myeloid leukemia had complete responses to venetoclax combined with hypomethylating agents, preliminary results from clinical trials have shown.

The anti-BCMA CAR T cell therapy bb21217 demonstrated an objective response rate of 83.3%, with a very good partial response or better rate of 75% in patients with heavily pretreated relapsed/refractory multiple myeloma.

An approach using machine learning to analyze genomic and clinical data from patients with myelodysplastic syndromes could replace the gold standard of predicting how long patients may live with the disease.

John P. Leonard, MD, associate dean for clinical research and Richard T. Silver Distinguished Professor of Hematology and Medical Oncology, Meyer Cancer Center, Weill Cornell Medicine and New York Presbyterian Hospital, discusses the key takeaway from the phase III AUGMENT trial, a randomized study of lenalidomide (Revlimid) plus rituximab (Rituxan) (R2) versus rituximab/placebo in patients with relapsed/refractory indolent non-Hodgkin lymphoma, during the 2018 ASH Annual Meeting.
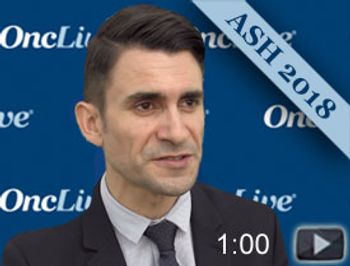
Jordan Gauthier, MD, MSc, senior clinical research fellow, Fred Hutchinson Cancer Research Center, discusses a study comparing efficacy and toxicity of CD19-specific chimeric antigen receptor (CAR) T cells alone or in combination with ibrutinib in patients with relapsed and/or refractory chronic lymphocytic leukemia (CLL) during the 2018 ASH Annual Meeting.

The CD3 and CD20 bispecific antibody mosunetuzumab demonstrated promising complete remission rates with tolerable toxicity for patients with relapsed/refractory B-cell indolent and aggressive non-Hodgkin lymphoma.

The triplet regimen of pembrolizumab (Keytruda) plus umbralisib and ublituximab reached a 90% response rate in patients with relapsed/refractory chronic lymphocytic leukemia.

Initial findings from the Beat AML study showed that rapid genetic testing in patients with AML was feasible and helpful, and that a precision medicine approach is possible for these patients, who must be treated urgently given the disease’s rapid progression.

Lisocabtagene maraleucel appeared tolerable and induced an 81.3% best overall response rate and 43.8% complete response rate in heavily pretreated, high-risk patients with chronic lymphocytic leukemia who previously received ibrutinib.

Single-agent ibrutinib continued to demonstrate strong results after 7 years of follow-up in both the frontline and heavily pretreated, relapsed/refractory setting for patients with CLL and SLL.

Two-year maintenance therapy with ixazomib led to a 39% improvement in progression-free survival compared with placebo in patients with newly diagnosed multiple myeloma who achieved a partial response to induction treatment with a proteasome inhibitor and/or an immunomodulatory agent following autologous stem cell transplant.

The combination of ibrutinib and venetoclax is well tolerated and eradicates minimal residual disease in the marrow after 12 months in 39% of patients with relapsed/refractory chronic lymphocytic leukemia.

The investigational agent zanubrutinib, a next-generation Bruton’s tyrosine kinase inhibitor, is highly active in patients with relapsed/refractory mantle cell lymphoma.

Checkpoint inhibition showed promise for augmenting or extending response to chimeric antigen receptor T-cell therapy in some patients with relapsed B-cell acute lymphoblastic leukemia.

Daratumumab added to bortezomib, lenalidomide, and dexamethasone induced at least a very good partial response in all patients and a stringent complete response or CR in 63% of patients at the end of consolidation therapy in the run-in phase of an open-label study of transplant-eligible patients with newly diagnosed multiple myeloma.

Receiving a stem cell transplant for the first time following CD19 CAR T-cell therapy induced a reduction in the risk for acute lymphoblastic leukemia (ALL) recurrence, according to a retrospective analysis of the phase I/II PLAT-02 study.

Farhad Ravandi, MBBS, professor of medicine, department of leukemia, The University of Texas MD Anderson Cancer Center, discusses a phase I first-in-human study of AMG 330, an anti-CD33 bispecific T-Cell engager (BiTE) antibody construct, in patients with relapsed/refractory acute myeloid leukemia (AML) during the 2018 ASH Annual Meeting.
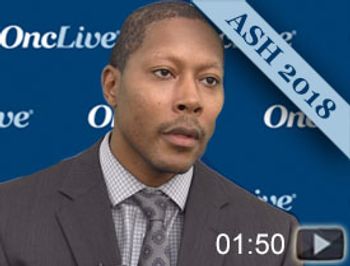
Tycel J. Phillips, MD, assistant professor, University of Michigan Cancer Center, discusses safety and efficacy findings for acalabrutinib plus bendamustine and rituximab in patients with treatment-naive or relapsed/refractory mantle cell lymphoma (MCL) during the 2018 ASH Annual Meeting.