
Epcoritamab was found to produce an encouraging overall response rate in patients with relapsed or refractory large B-cell lymphoma who previously received CAR T-cell therapy.

Your AI-Trained Oncology Knowledge Connection!


Epcoritamab was found to produce an encouraging overall response rate in patients with relapsed or refractory large B-cell lymphoma who previously received CAR T-cell therapy.
The addition of durvalumab and tremelimumab to chemotherapy led to encouraging responses and a suitable safety profile as neoadjuvant therapy in patients with advanced ovarian cancer, according to findings from the phase 2 KGOG 3046.

The addition of SOT101, an interleukin-2/IL-15 Rβγ superagonist, to Pembrolizumab generated a clinical benefit and encouraging safety data in patients with advanced solid tumors.

The addition of the selective oncolytic adenovirus CG0070 to pembrolizumab showed encouraging activity and safety in Bacillus Calmette-Guerin–unresponsive non-muscle invasive bladder cancer, according to early data from the phase 2 CORE1 trial.

The FDA has cleared an investigational new drug application for 177Lu-rhPSMA-10.1 as a potential therapeutic option for patients with metastatic castration-resistant prostate cancer.

The combination of the CD40 agonist antibody sotigalimab and pembrolizumab demonstrated a favorable safety profile and led to immunologic changes that correlated with clinical response in patients with unresectable stage III or IV metastatic melanoma.

The addition of the investigational anti-CD39 antibody TTX-030 to frontline treatment with the PD-L1 inhibitor budigalimab and mFOLFOX6 was determined to be safe and tolerable in patients with locally advanced or metastatic HER2-negative gastroesophageal junction adenocarcinoma.

The combination of nivolumab and ipilimumab demonstrated a statistically significant improvement in progression-free survival vs ipilimumab alone as second-line therapy in previously treated patients with stage III unresectable or stage IV melanoma.
The addition of BO-112 to pembrolizumab demonstrated efficacy and safety in patients with advanced melanoma who were resistant to anti–PD-1 therapies, according to final results from the phase 2 SPOTLIGHT203 trial.

MEDI5752 treatment led to a dose-dependent increase in peripheral T-cell proliferation and encouraging antitumor activity in patients with advanced solid tumors.
The combination of the novel adenoviral vector NG-641 and nivolumab is being investigated in patients in the phase 1a/b NEBULA trial in patients with previously treated metastatic or advanced epithelial tumors.

The FDA has placed a partial clinical hold on the open-label, dose-escalating phase 1/2 TakeAim Lymphoma study investigating the safety and efficacy of emavusertib in patients with relapsed or refractory B-cell malignancies.

The investigational Wee1 inhibitor ZN-c3 was found to be safe and to produce a disease control rate of 90.9% and an objective response rate of 27.3% in patients with advanced or recurrent uterine serous carcinoma.

The combination of ibrutinib and venetoclax administered for a fixed duration elicited durable responses and sustained progression-free survival in previously untreated, high-risk patients with chronic lymphocytic leukemia and small lymphocytic lymphoma, according to data from the phase 2 CAPTIVATE trial.

The novel KRAS G12C inhibitor, JDQ443, demonstrated early efficacy signals and a tolerable safety profile in initial data from the dose-escalation portion of the phase 1b/2 KontRASt-01 trial.

Perioperative pembrolizumab plus standard of care chemotherapy followed by adjuvant pembrolizumab showed a meaningful pathological complete response rate in patients with resectable gastric and gastroesophageal junction adenocarcinoma.
The combination of pepinemab and pembrolizumab elicited encouraging responses and tolerability as frontline therapy in patients with recurrent or metastatic head and neck cancer.
The CAR T-cell therapy BNT211, with or without an mRNA vaccine, produced efficacious and tolerable safety results.

The dual immunotherapy combination of nivolumab and ipilimumab elicited encouraging and durable responses with acceptable safety in patients with advanced or metastatic tumor mutational burden–high solid tumors that were refractory to standard therapies, meeting the primary end points of the phase 2 CheckMate-848 trial.

The oral ataxia-telangiectasia and Rad3–related protein inhibitor elimusertib produced durable and prolonged responses in patients with advanced solid tumors who have ATM gene alterations and BRCA1/2 defects.

In the ENDOLA trial, olaparib in combination with metronomic cyclophosphamide and metformin showed a significant non-progression rate in patients with recurrent advanced or metastatic endometrial cancer.

GD2-directed CAR T-cell therapy demonstrated prolonged periods of radiographic and clinical improvement in pediatric and young adult patients with H3K27M-mutated diffuse intrinsic pontine gliomas and spinal diffuse midline gliomas.

Sotorasib demonstrated an overall survival rate of 32.5% at 2 years in patients with KRAS G12C–mutant non–small cell lung cancer, according to longer follow-up data from the phase 1/2 CodeBreaK 100 trial.

Temferon, genetically modified Tie2-expressing monocytes targeting interferona2, showed the potential to activate the immune system and reprogram the tumor microenvironment in patients with glioblastoma.
Nivolumab in combination with a PD-L1/IDO peptide vaccine displayed encouraging efficacy and survival results among patients with metastatic melanoma.
Zanubrutinib monotherapy continues to demonstrate durable responses with an acceptable toxicity profile in patients with relapsed or refractory mantle cell lymphoma.
The China National Medical Products Administration’s Center for Drug Evaluation has granted a breakthrough therapy designation to relmacabtagene autoleucel for use in the treatment of patients with mantle cell lymphoma.

Venetoclax consolidation following first-line treatment with venetoclax and obinutuzumab did not reduce loss of minimal residual disease response vs MRD-guided venetoclax consolidation in previously untreated patients with chronic lymphocytic leukemia.
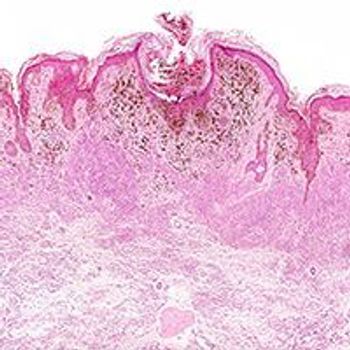
The combination of the oncolytic vaccine vusolimogene oderparepvec and nivolumab continued to drive encouraging responses with acceptable tolerability in patients with melanoma and other skin cancers.

The FDA has placed a partial clinical hold on the phase 1/2a TakeAim Leukemia trial, which is evaluating emavusertib as a single agent and in combination with azacitidine or venetoclax in patients with relapsed or refractory acute myeloid leukemia or high-risk myelodysplastic syndrome.