
R. Lor Randall, MD, discusses the intriguing risk-stratification–based trial in NSTRS and how the findings can be used to guide treatment decisions in this pediatric population.

Your AI-Trained Oncology Knowledge Connection!


Vice President of Content, OncLive and Cancer Network
Gina Mauro is your lead editorial contact for OncLive. She joined the company in 2015 and has held various positions on OncLive; she is also the on-air correspondent for OncLive News Network: On Location. Prior to joining MJH Life Sciences, she worked at Gannett as a full-time reporter with the Asbury Park Press. Email: gmauro@onclive.com

R. Lor Randall, MD, discusses the intriguing risk-stratification–based trial in NSTRS and how the findings can be used to guide treatment decisions in this pediatric population.

February 8, 2021 — Narsoplimab demonstrated clinical activity and a favorable survival benefit in patients with high-risk hematopoietic stem cell transplant-associated thrombotic microangiopathy, according to updated results of a pivotal phase 2 trial.

Synergizing EGFR TKIs with antiangiogenic agents and chemotherapy, as well as improving the use of molecular classification, are novel strategies aimed at taking the frontline setting for patients with EGFR-mutant non–small cell lung cancer to the next level.

Neil Berinstein, MD, discussed the early promise with this T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma.

Richard L. Schilsky, MD, FACP, FSCT, FASCO, sat down to share his proudest moments and obstacles throughout his career with ASCO, as well as advice for his successor, Julie Gralow, MD, who will step into the position on February 15, 2021.

Dan Costin, MD, discusses his strategies for patients with early-stage lung cancer and ongoing trials that could improve outcomes even further.

January 29, 2021 - Pembrolizumab plus ipilimumab did not improve survival and had higher rates of toxicity versus pembrolizumab monotherapy as a first-line treatment for patients with metastatic non–small cell lung cancer who had a PD-L1 tumor proportion score of 50% or greater, and did not harbor EGFR or ALK aberrations.

January 28, 2021 - Treatment with the KRAS G12C inhibitor sotorasib (formerly AMG 510) elicited a 6.8-month median progression-free survival in patients with KRAS G12C–mutated advanced non–small cell lung cancer.

January 28, 2021 - NRG1 fusions are detectable in patients with non–small cell lung cancer, providing a rationale to perform larger, retrospective studies assessing therapeutic outcomes in patients with NRG1 fusion–positive tumors and evaluate afatinib in this patient subgroup.

R. Lor Randall, MD, FACS, discusses the rationale and motivation behind tissue-engineered platforms and how they could significantly impact the understanding of how cancer arises in or metastasizes to bone.

David Cohn, MD, discusses how to approach COVID-19 vaccination in patients with cancer.

The idea that HPV-negative cervical cancer is possible, especially in a disease that is mainly driven by HPV positivity, is not a unanimous opinion.

Steven Devine, MD, discusses the non-relapse mortality data with Orca-T and how this technologic approach is changing how providers approach patients with hematologic malignancies following transplant.

January 17, 2021 — Ivosidenib tablets led to a 21% reduction in the risk of death compared with placebo in previously treated patients with IDH1-mutant cholangiocarcinoma, according to the final overall survival analysis of the phase 3 ClarIDHy trial.

Bemarituzumab combined with mFOLFOX6 demonstrated a 56% reduction in the risk of disease progression or death compared with placebo and mFOLFOX6 as a frontline treatment in select patients with FGFR2b-positive advanced gastric or gastroesophageal junction adenocarcinoma.

January 15, 2021 - Combination treatment with adjuvant S-1 and docetaxel led to an estimated 29% reduction in the risk of relapse compared with S-1 alone in patients with stage III gastric cancer.

Melissa Johnson, MD, shares her goals for the first 100 days as program director of the Lung Cancer Research at Sarah Cannon Research Institute, the current state of the anti-TIGIT antibody tiragolumab, and the future of lung cancer treatment.
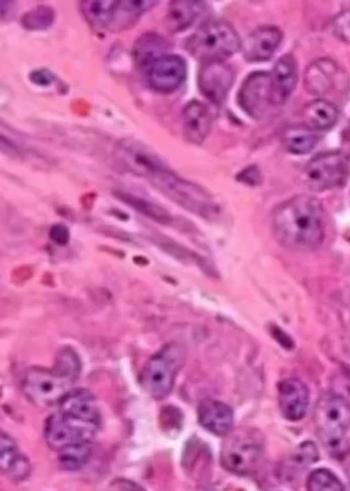
January 4, 2021 — The pivotal phase 2/3 RINGSIDE trial, which is evaluating the potent, selective oral gamma secretase inhibitor AL102, has been permitted to proceed and can potentially be used as a registrational study for adolescent and adult patients with desmoid tumors.

December 29, 2020 - The FDA has granted an orphan drug designation to Annamycin for the treatment of patients with soft tissue sarcomas.
Treatment with iodine (131I) apamistamab (Iomab-B) conditioning led to a 100% bone marrow transplant rate and engraftment in patients with active relapsed/refractory acute myeloid leukemia who are older than 55 years old, according to results of a single ad hoc interim analysis of the phase 3 SIERRA study.
The filing of a new drug application has been initiated with the FDA for surufatinib for the treatment of patients with pancreatic and non-pancreatic neuroendocrine tumors.

Nicholas C. Rohs, MD, highlights the influx of clinical trials and the addition of immunotherapy in the adjuvant setting of lung cancer.
December 22, 2020 — Enobosarm demonstrated clinical benefit at varying dose levels in patients with androgen receptor–positive, estrogen receptor–positive metastatic breast cancer.

December 21, 2020 — The 5-drug regimen of venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide showed a tolerable safety profile and encouraging antitumor activity with complete responses in patients with relapsed/refractory diffuse large B-cell lymphoma.

December 21, 2020 - The National Comprehensive Cancer Network has added 3 selinexor combination regimens to its Clinical Practice Guidelines in Oncology for previously treated patients with multiple myeloma.

R. Lor Randall, MD, FACS, discusses the concept and prospect of up-front resection in osteosarcoma from the Osteosarcoma Collaborative.

Balazs Halmos, MD discusses the year 2021 and how it will encompass a bigger focus on neoadjuvant and adjuvant trials, more novel molecular compounds for more select subgroups, and a migration of circulating tumor DNA into a more minimal residual disease–based setting.