
Increasing the dosage of lanreotide autogel from every 28 days to every 14 days led to encouraging progression-free survival outcomes in patients with progressive pancreatic and midgut neuroendocrine tumors.

Your AI-Trained Oncology Knowledge Connection!


Vice President of Content, OncLive and Cancer Network
Gina Mauro is your lead editorial contact for OncLive. She joined the company in 2015 and has held various positions on OncLive; she is also the on-air correspondent for OncLive News Network: On Location. Prior to joining MJH Life Sciences, she worked at Gannett as a full-time reporter with the Asbury Park Press. Email: gmauro@onclive.com

Increasing the dosage of lanreotide autogel from every 28 days to every 14 days led to encouraging progression-free survival outcomes in patients with progressive pancreatic and midgut neuroendocrine tumors.

Investigators are evaluating the combination of telotristat ethyl and Lutathera with a goal to improve progression-free survival in patients with well-differentiated neuroendocrine tumors in a randomized, phase 2 study that was highlighted during the 2020 NANETs Virtual Symposium.

The FDA has approved nivolumab (Opdivo) at 360 mg every 3 weeks plus ipilimumab (Yervoy) at 1 mg/kg every 6 weeks for the frontline treatment of adult patients with unresectable malignant pleural mesothelioma.
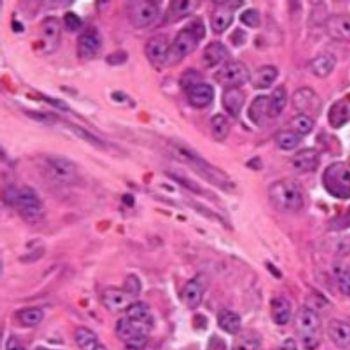
The presence of macroscopic metastases in regional lymph nodes is found to be related to poor survival outcomes in patients with sarcoma.

Patients with prostate cancer who harbor germline BRCA2 mutations were found to have significantly higher rates of somatic BRCA loss, somatic RB1 loss, and MYC amplification compared with non-carriers—factors that were independently associated with poorer survival outcomes.

Plinabulin was found to be a more favorable option for the prevention of chemotherapy-induced neutropenia compared with pegfilgrastim during the coronavirus disease 2019 pandemic.

R. Lor Randall, MD, highlights the innovative efforts by the COG, ongoing studies in sarcomas, and how the organization is adapting during a world with COVID-19.

The investigational CAR T-cell product AUTO3 in combination with pembrolizumab was found to have a tolerable safety profile and elicit durable complete responses in patients with relapsed/refractory diffuse large B-cell lymphoma.
The combination of the investigative PD-1 inhibitor camrelizumab plus the antiangiogenic agent apatinib led to early signals of clinical activity in patients with relapsed/refractory peripheral T-cell lymphoma, warranting further study.

Tisotumab vedotin demonstrated an objective response rate of 24% in patients with recurrent and/or metastatic cervical cancer who were previously treated with doublet chemotherapy and bevacizumab, if eligible.

Ipatasertib combined with abiraterone acetate plus prednisone led to a significantly superior radiographic progression-free survival and antitumor activity compared with placebo plus abiraterone/prednisone in patients with metastatic castration-resistant prostate cancer with PTEN loss.

BLU-945, an investigational precision therapy, elicited robust antitumor activity in multiple preclinical models of triple-mutated EGFR-positive non–small cell lung cancer .

First-line treatment with the third-generation ALK TKI lorlatinib significantly improved progression-free survival, and also was associated with higher overall and intracranial response rates, compared with crizotinib in patients with ALK-positive non–small cell lung cancer.

Treatment with sacituzumab govitecan led to a 59% reduction in the risk of disease progression or death compared with physician’s choice of single-agent chemotherapy in patients with previously treated metastatic triple-negative breast cancer.



The investigative PD-1 inhibitor balstilimab as a single agent and combined with the CTLA-4 inhibitor zalifrelimab showed promising objective response rates, regardless of PD-L1 expression, and a tolerable safety profile in patients with recurrent/metastatic cervical cancer.

The combination of nivolumab and cabozantinib led to a 49% reduction in the risk of disease progression or death, while also significantly improving overall survival and doubling objective response rate, compared with sunitinib in the first-line treatment of patients with advanced renal cell carcinoma.

Frontline maintenance treatment with avelumab plus best supportive care improved both progression-free and overall survival compared with BSC alone across prespecified subgroups of patients with advanced or metastatic urothelial carcinoma that has not progressed on first-line platinum-based chemotherapy.

The PD-1 antibody cemiplimab established encouraging clinical activity in patients with locally advanced basal cell carcinoma who progress on or are intolerant to hedgehog inhibitors, regardless of PD-L1 expression.

Patients with mantle cell lymphoma who have high-risk biology, including blastoid variant, a Ki-67 score of at least 30%, or high p53 expression, had a significantly shorter failure-free and overall survival.