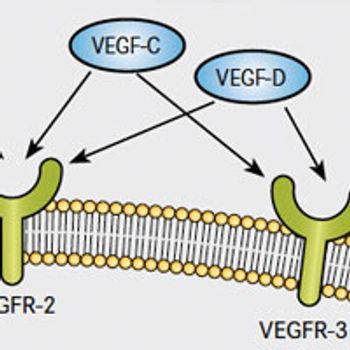
Early expectations of antitumor activity across all cancer types have been tempered by clinical disappointment in many cases and only modest efficacy in others. Nonetheless, VEGF signaling continues to present a promising target.

Your AI-Trained Oncology Knowledge Connection!


Early expectations of antitumor activity across all cancer types have been tempered by clinical disappointment in many cases and only modest efficacy in others. Nonetheless, VEGF signaling continues to present a promising target.
Genome sequencing studies are beginning to match distinct genomic profiles to different subtypes of kidney cancer, driving a dramatic shift in our understanding of these diseases and how to treat them.

New research is carving out a role for ipilimumab and a second CTLA-4-targeting drug, tremelimumab, as combination therapy with potential in a variety of solid tumors. In addition to combinations with other immune checkpoint inhibitors, the agents are being evaluated across a breadth of other strategies.

Mark Socinski, MD, discusses the three generations of drugs are now available to treat patients with EGFR-mutant non–small cell lung cancer, and mutation testing for patients diagnosed with advanced or metastatic disease has been incorporated into clinical practice guidelines.

Experts in the field assert that the path forward requires a paradigm shift toward integrative analyses that encompass multiple classes of genomic aberrations and consensus classification of CRC based on genomic data to facilitate more effective management of this disease.
After a rocky start, drugs that inhibit the epidermal growth factor receptor pathway have evolved into a new treatment paradigm for patients with non–small cell lung cancer whose tumors harbor EGFR mutations.
With technological advancements in genome sequencing, researchers are now gaining a more detailed picture of the genetic drivers of cervical cancer and the important role that the human papillomavirus, responsible for the vast majority of cervical cancer cases, plays in molding the genetic profile of this disease.
It is becoming increasingly clear that PD-1/ PD-L1 and CTLA-4 represent just the tip of the iceberg when it comes to manipulating the immune system to fight cancer, and the number of known checkpoints—and with it the list of potential drug targets—has expanded in recent years.
Amid a growing understanding about the role of epigenetics as a driver of cancer, researchers have turned their attention to a key player in the process: histone deacetylases.
Particular progress has been made in the development of small-molecule inhibitors of key signaling pathways that are responsible for the unique characteristics of stem cells, developed by biotechnology companies focused solely on CSC-targeting therapies.
The Hedgehog pathway is one of several key developmental cell-signaling networks whose aberrant activation in adult tissues can lead to cancer.

An improved understanding of the natural history of HPV, its interaction with the host immune system, and the distinct molecular alterations underlying HPV-positive cancers, are fueling development of new drugs, particularly immunotherapies geared toward generating an HPV-specific immune response.
Drug developers are moving forward with scores of new agents that may cross the blood brain barrier in several classes of therapy including chemotherapies, molecularly targeted agents, and immunotherapies.

Jennifer A. Woyach, MD, discusses examining the role of BTK inhibition in CLL using murine and cellular models and mechanisms of resistance to BTK inhibitor therapy.

Combination therapy has significant potential to fill the niche of developing resistance to BTK inhibitors and rational pairings of ibrutinib with other standard treatments, including chemoimmunotherapy and CD20-targeting monoclonal antibodies (mAbs) are rapidly gaining ground.

With increased understanding of the biology of CRPC and the mechanisms of action of AR-targeting drugs, researchers are developing a growing appreciation for the extensive heterogeneity and complexity of both prostate cancer and androgen signaling.
The prospects for mAbs to change the treatment paradigm for multiple myeloma have grown considerably brighter as early-phase clinical trial results suggest that emerging agents with novel mechanisms of action are capable of delivering significant efficacy.

Josh D. Lauring, MD, PhD, of Johns Hopkins Medicine, discusses efforts to develop breast cancer therapies by targeting the PI3K pathway.
As we enter an era of unprecedented clinical trial activity in gastric cancer, with thousands of patients enrolled or set to be enrolled in large, randomized phase III trials of novel targeted agents, we may finally be on the path to changing the course of this disease.

Amid continuing excitement over the potential for PD-1 pathway immune checkpoint blockade strategies in anticancer therapies, research presented at the 2014 American Society of Hematology (ASH) Annual Meeting helped established a foundation for the use of anti- PD-1/PD-L1 agents in hematologic malignancies.
Agents that target components of the programmed death-1 (PD-1) pathway are poised to make an impact on the treatment of patients with hematologic malignancies, particularly Hodgkin lymphoma (HL).

As one of the most significant predictors of hereditary breast and ovarian cancer, the BRCA1/2 genes have become the poster child for genetic testing, thrust into the limelight by a high-profile court battle and a celebrity's disclosure.

Despite numerous reports of "miracle cures" for a select few patients treated with a variety of different anticancer therapies, drugs that failed to show improvement for a large number of patients have historically been considered failures and have been shelved.
The 5-year survival rate for patients with metastatic colorectal cancer (CRC) remains dismal.

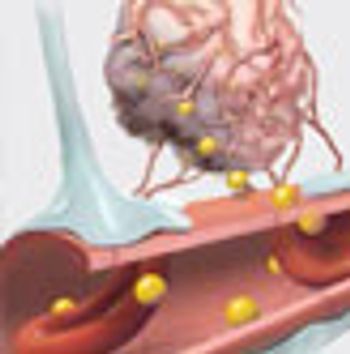
Despite being discovered more than 150 years ago, tumor cells present in the blood of patients with cancer are only now inspiring significant research efforts. Technological advancements have allowed the isolation and enrichment of these rare cells and, as potential metastatic "emissaries," they have significant potential for improving the detection and treatment of advanced and possibly even early-stage disease.
A decade after bevacizumab (Avastin) debuted as the first anticancer therapy to target angiogenesis, new strategies to attack this hallmark of cancer continue to be a major research focus, resulting in the development of novel agents and fresh treatment settings for existing drugs.
The concept of targeting mitotic cell division to halt the progression of rapidly dividing cancer cells has long been a staple of oncology therapy, yet chemotherapy agents that are the prime examples of this approach are nonselective in their action and can kill normal and malignant cells alike.
Although hormone-targeting strategies have been a mainstay of prostate and breast cancer therapies for decades, an improved understanding of the mechanisms underlying these malignancies has led researchers to focus fresh attention on the complex activity of the androgen receptor (AR) as a point of attack.
Hailed as "new ammunition in the war against cancer" and featured in TIME magazine at the turn of the new millennium, molecularly targeted therapies have gone on to revolutionize cancer treatment. Clinical responses, however, are all too often short-lived as cancer cells become resistant.
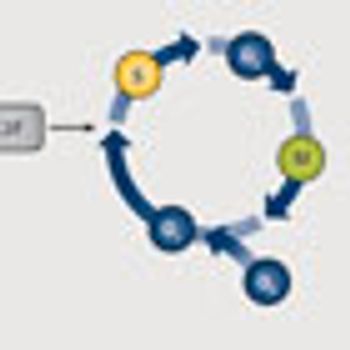
As gatekeepers of the cell cycle, the cyclin-dependent kinases are often implicated in the progression of cancer and make prime targets for therapy.