Articles by Jane de Lartigue, PhD

Cancer cells must maintain a delicate balance to prevent catastrophic levels of DNA damage from triggering cell death, and their heavy reliance on the remaining normal DNA damage response components creates a therapeutically targetable Achilles’ heel.
As researchers gain a better understanding of the unique aspects of individual tumor types and their surrounding microenvironment, the design of novel therapies categorized as prodrugs is become increasingly sophisticated, and several novel constructs show particular promise.

Although CD19 has proved to be an attractive and effective target for chimeric antigen receptor T-cell therapies in hematologic malignancies, a significant subset of patients treated with this groundbreaking form of immunotherapy eventually relapse.

Amid a pressing need to identify patients most likely to respond to immune checkpoint inhibitors, tumor mutational burden has emerged as a highly promising and clinically validated biomarker.

Despite gaps in current knowledge, investigators are already working toward the development of novel treatment strategies to combat the effects of ESR1 mutations.

Recently, immuno-oncologists have turned their attention to the role of the second arm of the immune response—the more rough-and-ready innate arm, which serves as the body’s frontline defense against pathogenic invaders and, it seems, cancer.

Anthony G. Letai, MD, PhD, discusses the complex nature of apoptosis and efforts to target the process.

Myeloid cell leukemia 1 has emerged as an intriguing target for anticancer drug development as researchers turn their attention to strategies directed at evasion of apoptosis.

Alice T. Shaw, MD, PhD, discusses the challenge of resistance mechanisms in the treatment of patients with non–small cell lung cancer whose tumors harbor ALK rearrangements.
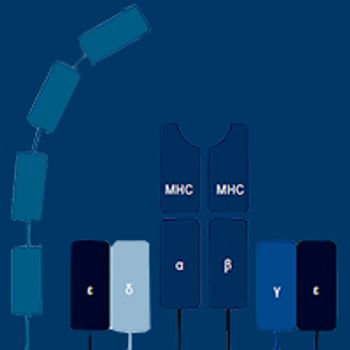
Components of the T-cell receptor complex, which links antigen recognition with T-cell activity and effector function, are being exploited for several types of cancer immunotherapy.

Srdan Verstovsek, MD, PhD, discusses the rationale for targeting JAK signaling, new drugs under study for this pathway, and clinical challenges associated with these therapies.
A growing appreciation of the role of the tumor microenvironment in fostering the development of malignancies is prompting the pursuit of anticancer therapies that target components of this supportive niche as opposed to the tumor itself.
Monoclonal antibodies that have the ability to selectively target cancer cells by binding to specific tumor-associated antigens have carved out an important role in anticancer therapy.

ALK inhibitors have followed a rapid trajectory from bench to bedside, taking over from chemotherapy as standard frontline treatment for patients with advanced non– small-cell lung cancer with ALK gene rearrangements.

Dysregulation of the JAK pathway plays a role in the development of numerous tumor types; it is particularly central to the pathophysiology of myelofibrosis and has long been recognized as a potentially valuable therapeutic target in that malignancy.

Chimeric antigen receptor (CAR) T cells have hit prime time, with the 2 recent FDA approvals for this class of cell-based therapy.

Mutations in the BRAF kinase are found in a relatively small number of patients with metastatic colorectal cancer, but they nonetheless have important implications for prognosis and response to standard therapy.

David S. Hong, MD, discusses the tropomyosin receptor kinase pathway and potential new molecularly targeted therapies.

In its fight against pancreatic cancer, the Pancreatic Cancer Action Network aims to improve current survival and double survival by 2020.
There is an urgent need for new approaches, particularly since pancreatic cancer is expected to become the second leading cause of cancer-related mortality by 2030.

Although dual HER2 blockade strategies have become an important part of the treatment paradigm for patients with HER2-positive breast cancer, the complexities of administering these therapies continue to unfold.

Although drugs that target HER2 have transformed the prognosis for many patients with breast cancer, the development of resistant disease remains a significant clinical challenge.

The growing use of next-generation sequencing has only recently revealed the neurotrophic tropomyosin receptor kinase (NTRK) gene presence across a wide range of tumor types and piqued interest in their potential as anticancer targets.

Many challenges remain to the optimal interpretation of genetic test results and the effective translation of these data into improved diagnostic, prognostic, and therapeutic capabilities.

Jeff Sharman, MD, discusses the prospects for progress in targeting elements of the BCR pathway.
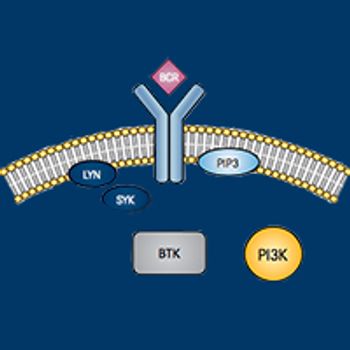
Despite the availability of numerous effective treatment options, most patients with B-cell malignancies still experience frequent relapses and progressively shorter remissions, creating a pressing need for new drugs to add to the therapeutic arsenal.
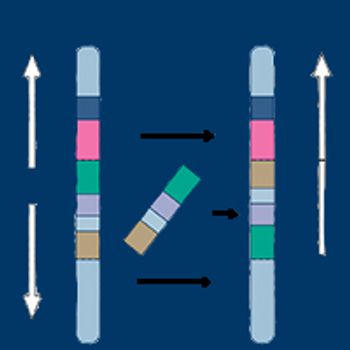
Thus far, only a small portion of known gene fusions have been tested with functional assays in an effort to understand if, and how, they drive cancer. Newly identified and well-established gene fusions alike continue to provide promising therapeutic targets and broaden our understanding of cancer development.
The past several decades have witnessed a dramatic improvement in the treatment of patients with multiple myeloma, the second most common type of hematologic malignancy. A better understanding of the biology of this disease and the introduction of a wealth of novel drug classes has more than doubled median survival times.
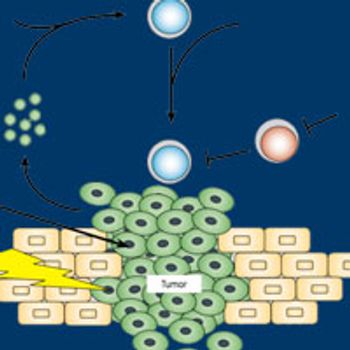
Promising reports of preclinical and early clinical data in 2016 are poised to further boost the development of rational combinations of OX40 agonists with checkpoint immunotherapies, surgical resection, radiotherapy, and even the potential for 3-drug cocktails.
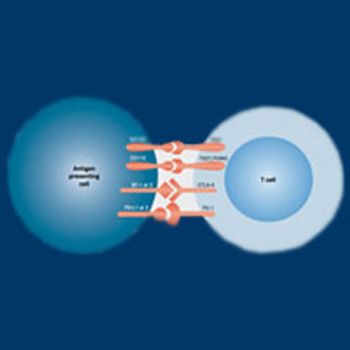
As researchers continue to identify a growing number of immune checkpoints as targets for anticancer therapy, the recently discovered TIGIT pathway is emerging as a promising new avenue for exploration.























