B7-H3, a member of the same protein family as PD-L1, has garnered attention as one of a slew of alternative targets riding the wave of success of immune checkpoint inhibitors in cancer immunotherapy.

Your AI-Trained Oncology Knowledge Connection!


B7-H3, a member of the same protein family as PD-L1, has garnered attention as one of a slew of alternative targets riding the wave of success of immune checkpoint inhibitors in cancer immunotherapy.
Novel strategies to modulate the microbiota are now an area of robust investigation

Mutations on the ESR1 gene, which encodes the estrogen receptor, have emerged as an important driver of resistance to endocrine therapies, which form the backbone of treatment for patients with ER-positive, HER2-negative breast cancer.
A growing recognition of the distinct clinical, pathological, and biological features of lung cancers that arise in nonsmokers is fostering greater interest in examining the molecular underpinnings of lung cancer in this patient subset.
Primary and metastatic brain tumors present a significant therapeutic challenge, in large part because they are protected by the blood-brain barrier, a highly restrictive interface between the bloodstream and the brain that prevents most drugs from accessing the brain parenchyma.
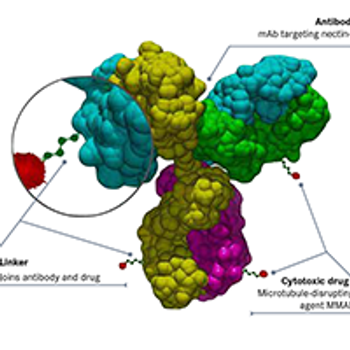
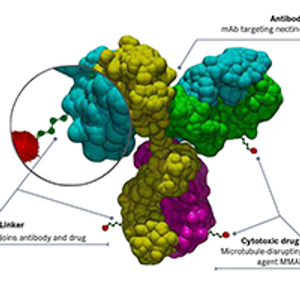
Most ongoing clinical trials exploring nectin-4 as a target involve studies of enfortumab vedotin and its potential synergy with immune checkpoint inhibitors in bladder cancer, while several other early-phase studies are testing novel agents in solid tumors.
The understanding of EGFR signaling in non–small cell lung cancer continues to evolve, helping to spark the development of novel therapies for new patient populations with uncommon alterations.
The lead novel candidate, the WEE1 inhibitor adavosertib, has been tested in more than 50 completed or ongoing clinical studies but has yet to proceed to a phase 3 trial despite showing promising safety and efficacy as monotherapy and in combination with a range of other cancer therapies.
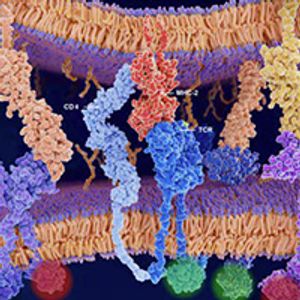
During the past decade, a growing number of PD-1/PD-L1 inhibitors gained FDA approval to treat a wide range of cancer types. Their stimulatory counterparts also emerged as sought-after anticancer targets but have proved much more challenging to manipulate therapeutically.

Three-quarters of all cases of lung adenocarcinoma, the most common type of non–small cell lung cancer, are defined by oncogenic driver events involving receptor tyrosine kinase–orchestrated cellular signaling pathways.
Among the pioneering targets for antibody therapy was CD20, the pursuit of which ultimately led to the first FDA-approved mAb for cancer therapy, rituximab, and defined a new era in the management of B-cell malignancies.

Frequently dysregulated in cancer cells, the PI3K pathway has long been a high-priority therapeutic target in oncology. However, initial efforts with pan–class I PI3K inhibitors were hampered by disappointing efficacy and substantial toxicity.
The curative potential of allogeneic hematopoietic stem cell transplantation for many hematologic malignancies is hindered by the frequent development of graft-vs-host disease, a potentially fatal complication resulting from a complex interaction between donor immune cells in the graft and the host’s immune system.
Although anticancer therapies that leverage T cells have commanded the most attention in the immuno-oncology era of the past decade, strategies based on natural killer cells have recently emerged as attractive approaches.
Although endocrine therapies have revolutionized the treatment of breast cancers driven by the estrogen receptor, the development of resistance remains a major challenge that limits long-term remission with currently available drugs.
Although Ki-67 is a commonly used measure of cellular proliferation in breast cancer tissue, its utility as a biomarker for helping to guide therapy decisions has been clouded by technical and clinical questions.

The identification of oncogenic driver mutations in non–small cell lung cancer to inform targeted therapy selection is the bedrock of clinical practice in this disease, with current estimates suggesting that more than half of patients harbor an actionable mutation.
Over the past 2 decades, a growing number of targetable tumor-specific molecular alterations have been identified, ushering in the era of precision oncology. Now, alterations in the RET gene can be added to the list of druggable targets.

Despite decades of research and drug development, however, the therapeutic utility of targeting RAS is limited to ruling out treatment with certain drugs in patients without RAS mutations.

November 24, 2020 - Until now, the field of cell-based immunotherapy has been dominated by chimeric antigen receptor (CAR) T cells, with groundbreaking FDA approvals for 3 drugs across several types of hematologic malignancies. In solid tumors, however, CAR T-cell therapies have yet to gain ground.

The identification of chromosomal rearrangements that result in oncogenic gene fusions ushered in the era of molecularly targeted therapies in oncology.

Investigators are developing a new way to target a key oncogenic mechanism that may prove to be an effective anticancer strategy, particularly against hematologic malignancies.
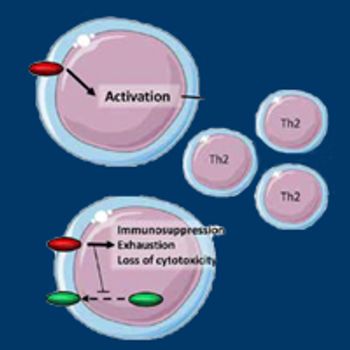
TIGIT, an inhibitory immune checkpoint that plays a central role in limiting antitumor responses, is attracting robust interest in the research community as a novel target for combination therapies across a range of cancer types, particularly solid tumors.
Precision medicine advancements are opening a new chapter in the development of anticancer therapies that target the HER2 pathway, resulting in 3 approvals for breast cancer in less than a year and raising hopes for attacking other cancer types.
Considerable efforts have focused on developing agonists of costimulatory receptors, including OX40.
Homologous recombination, one of the major mechanisms of defective DNA repair, has emerged as a bona fide therapeutic target, yet its optimal use as a biomarker for patient selection remains a clouded scientific question.
A novel compound that uses abundant lipids in cancer cell membranes to deliver a radioisotope to the tumor environment shows early signs of efficacy in a range of B-cell malignancies.

During the past 5 years, therapies targeting CD38, a protein highly expressed on the surface of plasma cells, have helped fuel the rapidly growing treatment options for patients with multiple myeloma.
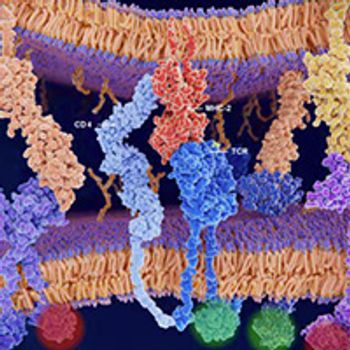
Over the past decade, immunotherapy has established itself as one of the pillars of cancer treatment, thanks in large part to the success of monoclonal antibodies that target the immune checkpoint protein PD-1 or its main ligand, PD-L1.

Published: April 16th 2021 | Updated:
Published: January 2nd 2022 | Updated:
Published: June 8th 2020 | Updated:
Published: June 16th 2021 | Updated:
Published: June 10th 2022 | Updated:
Published: July 16th 2021 | Updated: