Articles by Jane de Lartigue, PhD
Even as technological advances make it possible to sequence DNA on a large scale at relatively lower costs and in shorter time-frames, emerging evidence from the world's top research laboratories suggests that scientists are still a long way from having a complete catalog of cancer genes.

An interview with Renier J. Brentjens, MD, PhD, on new therapies for patients with acute and chronic leukemias, in particular novel immunotherapies such as chimeric antigen receptor T cells.
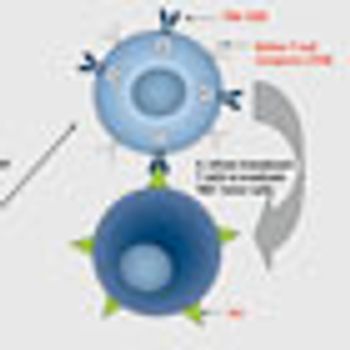
For the past two decades, researchers have been exploring B-cell specific antigens in hopes of developing a new anticancer target that would mirror the success of the CD20-targeting rituximab (Rituxan). Now, strategies aimed at CD19 are proving particularly promising.

Five early-phase clinical trials exploring chimeric antigen receptor (CAR) T-cell therapy have been suspended temporarily in response to the deaths of 2 patients with adult B-cell acute lymphoblastic leukemia
Amid a growing recognition of the need to improve the process of developing oncology drugs, the novel I-SPY 2 clinical trial in breast cancer has demonstrated the potential to deliver new, effective treatment options more rapidly to patients who would most benefit while dramatically reducing the time and costs currently required to evaluate experimental therapies.

Although breakthrough successes are generating a renaissance for anticancer immunotherapies, the framework for translating promising research results into clinical practice remains very much under construction.
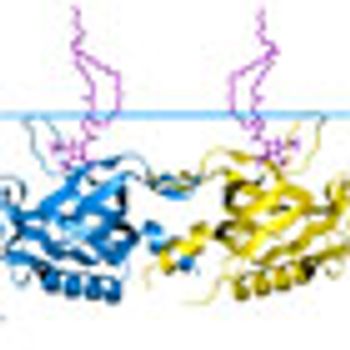
A greater understanding of the molecular mechanisms underlying lymphoid malignancies has fostered the development of targeted therapies, including those aimed at B-cell signaling pathways.

An interview with Barry D. Nelkin, PhD, from Johns Hopkins, on his research investigating a promising new target in a KRAS-related pathway, cyclin-dependent kinase 5.
The members of the RAS oncogene family are central cogs in many different cell-signaling pathways, coordinate a variety of important cellular processes, and are highly mutated in a number of different cancers, including several with extremely poor prognosis.
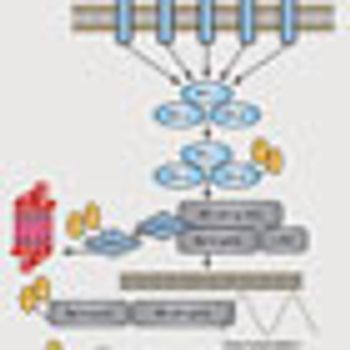
The cytokine receptor CD30 was identified as an attractive anticancer target more than 30 years ago

Although immunotherapy advances in solid tumors have captured much attention in recent years, therapeutic strategies that enable the patient's own immune system to battle cancer cells have long been integrated into the treatment of patients with hematologic malignancies.
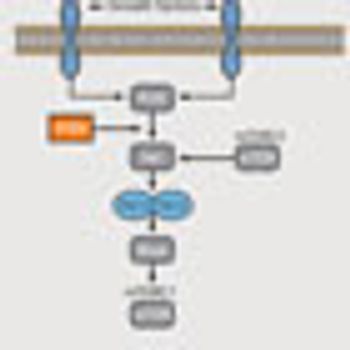
The phosphatidylinositol 3-kinase pathway is frequently deregulated in cancer at multiple different points, and has therefore emerged as one of the most deeply explored cell-signaling networks in oncogenic research.
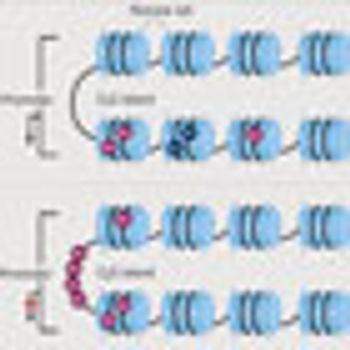
Several epigenetic therapies are already approved by the FDA, and many more are in the preclinical investigation and clinical trial phases. More than 100 agents are in various stages of development, and the field of epigenetics holds exciting implications for cancer detection, treatment, and prognosis.
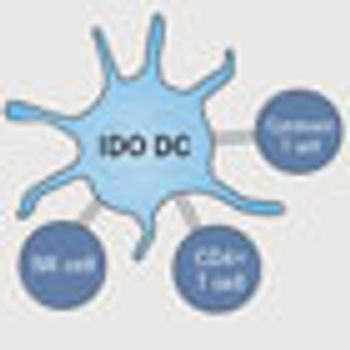
IDO is a key enzyme in the normal regulation of the host's adaptive immune response. Its role in regulating the immune response was initially demonstrated when pregnant mice were given IDO inhibitors, resulting in the rejection of the unborn fetus by the maternal immune system.

Douglas Hanahan and Robert Weinberg acknowledged the importance of the immune system in cancer development in 2011, when they added immune evasion to their list of "hallmark" abilities that are essential for the transformation of normal cells into cancerous ones.
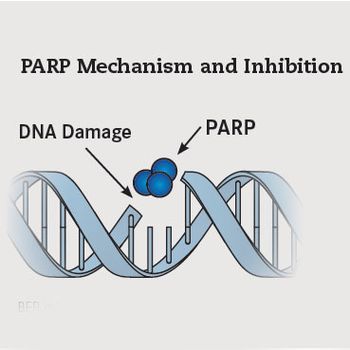
Anticancer agents that inhibit the poly (ADP-ribose) polymerase (PARP) family of enzymes have defined a new therapeutic paradigm known as synthetic lethality.

An interview with Andrew Scott, MB,BS, MD, whose work has led to the successful translation of novel potential cancer therapeutics into clinical development.
More than a century has passed since the discovery of antibodies and thanks to a number of Nobel Prize-winning scientists, we have begun to realize their potential as therapeutic tools in cancer.
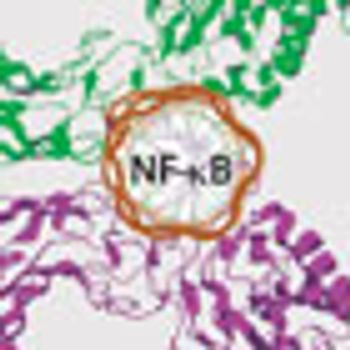
The complex regulation of NF-κB activation has presented significant challenges for the development of anticancer agents, but researchers are now beginning to better understand and embrace this complexity to drive development of a variety of novel NF-κB-targeting strategies.

Jeanette H.W. Leusen, PhD, focuses on studying the working mechanisms of therapeutic antibodies and the biology of fragment crystallizable receptors, including the anti-CD20 monoclonal antibody rituximab in patients with non-Hodgkin lymphoma.
A large proportion of patients become rituximab-refractory, which has prompted the development of newer CD20 agents with altered structures that are designed to improve upon rituximab's performance.
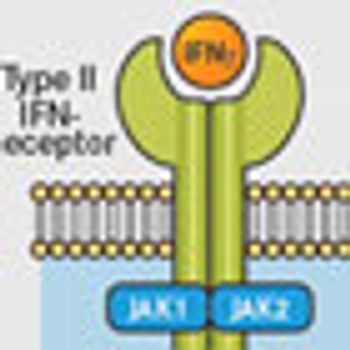
Interferons have evolved from a "cure-all" for cancer, as they were initially touted, to a more tempered yet equally vital role in treating a number of different disease states, including many different types of cancer.

For more than 20 years, Ahmed Lasfar, PhD, has been exploring the cell-signaling activity of interferons and he has been involved in the characterization of a new type of interferon, IFNλ, and its antitumor activity.

Benjamin W. Purow, MD, a researcher whose focus is on glioblastomas, discusses the Notch pathway and the development of Notch-targeted anticancer agents.
Notch-targeted agents that were initially intended for the treatment of Alzheimer disease are now being examined for their possible anticancer activity.

Suzanne L. Topalian, MD, has led clinical development of monoclonal antibodies to treat patients with melanoma and other solid tumors, including those targeting the PD-1 T cell co-receptor.
Increasing evidence suggests that the ability to outsmart the body's immune response represents a hallmark of tumor development.
Though located far downstream of the extracellular trigger that initiates its signaling pathway, the MEK protein is no less significant a player in the cascade of events that promotes key cellular processes.
Two leading researchers discuss the role of the ALK signaling pathway and development into new ALK-targeted anticancer therapies.
There has been stunning progress as preclinical findings of the ALK gene in patients with lung cancer were rapidly translated into the availability of an FDA-approved therapeutic ALK inhibitor, crizotinib.





























