Genitourinary Cancers
Latest News
Latest Videos

CME Content
More News

OncLive interviewed experts at the State of the Science Summits™ in December 2019 on what the biggest advances in oncology were in 2019.

Japan’s Pharmaceuticals and Medical Devices Agency has approved the combination of pembrolizumab and axitinib for the first-line treatment of patients with radically unresectable or metastatic renal cell carcinoma.

The FDA has granted a priority review designation to a new drug application for UGN-101 for the treatment of patients with low-grade, upper tract urothelial cancer.

The FDA has approved enfortumab vedotin-ejfv for the treatment of adult patients with locally advanced or metastatic urothelial cancer who have received prior treatment with a PD-1/PD-L1 inhibitor and platinum-containing chemotherapy.

Robert J. Motzer, MD, discusses the current frontline treatment landscape of metastatic renal cell carcinoma amongst an influx of approvals.
Martin H. Voss, MD, discusses results of a randomized phase I/II trial looking at the combination of MEDI0680 and durvalumab (Imfinzi) versus nivolumab (Opdivo) in metastatic renal cell carcinoma.

The current management of metastatic renal cell carcinoma reflects the progress of medical oncology throughout the past several decades. The standard of care has evolved from cytokine-based therapies to targeted molecular therapies and immune checkpoint inhibitor–based strategies.

Kathryn E. Beckermann, MD, PhD, instructor of medicine, Division of Hematology/Oncology, Department of Medicine, Vanderbilt University Medical Center, discusses sequencing strategies in metastatic renal cell carcinoma (mRCC).

Nancy B. Davis, MD, discusses the results of the phase III IMvigor130 trial in metastatic urothelial cancer.

Kathryn E. Beckermann, MD, PhD, discusses the available single-agent and combination options for the treatment of patients with metastatic renal cell carcinoma.

Immunotherapy prescriptions for patients with metastatic bladder cancer declined rapidly after the FDA altered the indications of 2 drugs, suggesting that oncologists can keep pace with changes associated with the agency’s accelerated approval program.
Erik P. Castle, MD, discusses minimally invasive surgical approaches in renal cell carcinoma.

As the first targeted therapy in bladder cancer, erdafitinib (Balversa) expands the limited treatment options for the subset of patients with urothelial carcinoma.

Erik P. Castle, MD, discusses the role of cytoreductive nephrectomy in renal cell carcinoma based on interpretation of the CARMENA trial results.

Thai H. Ho, MD, PhD, discusses frontline treatment considerations in metastatic renal cell carcinoma, the utility of TKI monotherapy, and new combinations on the horizon.
Eric Jonasch, MD, discusses how to modulate the tumor microenvironment in renal cell carcinoma.
Thai H. Ho, MD, PhD, discusses the role of TKI monotherapy in the first-line treatment of patients with metastatic renal cell carcinoma.

David F. McDermott, MD, discusses the combination of VEGF TKIs and PD-1 inhibitors in advanced renal cell carcinoma.

Three game-changing immunotherapy trials have transformed the treatment paradigm in frontline clear cell renal cell carcinoma. At the 37th Annual CFS®, Robert J. Motzer, MD, compared the findings from the three studies.

Robert J. Motzer, MD, discusses the first-line treatment landscape of metastatic clear cell renal cell carcinoma.

Guru P. Sonpavde, MD, discusses the safety profile of erdafitinib, which was approved by the FDA in April 2019 to treat adult patients with locally advanced or metastatic bladder cancer with an FGFR3 or FGFR2 alteration that has progressed on platinum-containing chemotherapy.
Thomas Powles, MD, MBBS, MRCP, professor, Genitourinary Oncology, lead for Solid Tumour Research, and director, Barts Cancer Institute, discusses the design and findings of the phase Ib BISCAY trial in platinum-refractory urothelial cancer.

The European Commission has approved the combination of avelumab and axitinib for the frontline treatment of adult patients with advanced renal cell carcinoma.
Guru P. Sonpavde, MD, discusses current neoadjuvant immunotherapy options in bladder cancer based on cisplatin eligibility status.
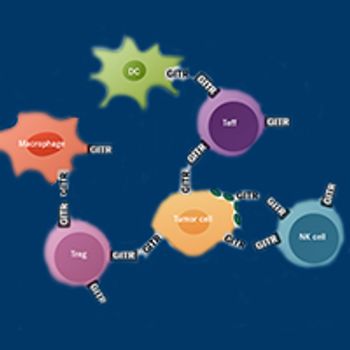
Immunotherapies designed to exploit the host immune system to specifically target cancer cells exploded onto the oncology scene in the mid-1980s, when the first such agents started to show success in melanoma and renal cell carcinoma.