Gynecologic Oncology
Latest News
Pre- and Co-administration of Nivolumab Proves Safe in Locally Advanced Cervical Cancer Treated With CCRT
Latest Videos
CME Content
More News

Julian Schink, MD, discusses a study evaluating racial differences in the mutational landscape of serous endometrial cancer, underscores the need for appropriate genomic testing and treatment for Black women with the disease, and explains the importance of racial representation across clinical trials.
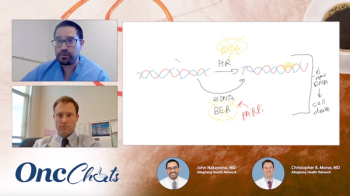
In this second episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss how to counsel patients with ovarian cancer whose tumors harbor BRCA mutations.

The FDA has expanded its approval of the VENTANA MMR RxDx panel to identify patients with mismatch repair–deficient solid tumors and as a companion diagnostic assay to determine eligibility for pembrolizumab as a treatment for patients with mismatch repair–proficient endometrial cancer.
In this first episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss how BRCA mutational status affects treatment decisions for patients with ovarian cancer.

Avelumab plus talazoparib showcased a favorable toxicity profile and overall modest activity in patients with mismatch repair–proficient endometrial cancer; however, immunogenomic profiling revealed a certain subset who might derive benefit from the doublet, warranting further investigation.

TogetHER for Health is honoring the contributions of the University of Alabama at Birmingham’s Isabel Scarinci, PhD, with the first-ever Trailblazer Award for her tireless dedication to saving women’s lives both in Alabama and across the world.

The combination of ofranergene obadenovec and paclitaxel failed to elicit a statistically significant improvement in progression-free survival or overall survival compared with paclitaxel alone in patients with platinum-resistant ovarian cancer, missing the coprimary end points of the OVAL trial.

Dr Monk discusses pertinent efficacy and safety data from the ATHENA-MONO trial, which evaluated first-line maintenance treatment with rucaparib in patients with stage III-IV high-grade ovarian cancer.

Patients with platinum-resistant ovarian cancer have historically been an underserved population with few effective treatment options.
Socazolimab produced encouraging response rates with an acceptable safety profile in patients with recurrent or metastatic cervical cancer, irrespective of PD-L1 status, according to findings from the dose-expansion portion of a phase 1 trial.
The combination of anlotinib and sintilimab was found to provide a long-term survival benefit to patients with previously treated advanced cervical cancer.

The combination of niraparib and dostarlimab produced a low overall response rate in patients with platinum-resistant ovarian cancer without a known BRCA mutation who had progressed and received prior bevacizumab, one that did not reach the threshold for second-stage accrual to the phase 2 MOONSTONE/GOG-3032 trial.

Robert Wenham, MD, MS, FACOG, FACS, discusses the current treatment landscape for patients with cervical and endometrial cancers, ADCs and PARP inhibitors in ovarian cancer, advancements in HER2-positive breast cancer, and surgical options for patients with ovarian cancer.
The antibody-drug conjugate farletuzumab ecteribulin demonstrated notable antitumor activity with a manageable safety profile in patients with platinum-resistant ovarian cancer.

Maurie Markman, MD, offers expert insights on the hurdles facing community oncologists, the uptake of biomarkers in the clinic, and an overview of what to look forward to in his area of expertise: gynecologic oncology.
The combination of abemaciclib and fulvestrant led to encouraging responses when used as neoadjuvant therapy which translated to high complete gross resection rates in patients with advanced, low-grade serous ovarian, fallopian tube, and peritoneal carcinoma.

Single-agent dostarlimab generated durable antitumor activity in patients with advanced or recurrent endometrial cancer with mismatch repair deficient/microsatellite instability–high or mismatch repair proficient/mismatch stable disease.
Selinexor generated a longer median progression-free survival compared with placebo in patients with advanced or recurrent endometrial cancer, including those with p53 wild-type tumors.
OncLive® will be LIVE with OncLive® News Network: On Location at the 2022 ASCO Annual Meeting. Each day, we will broadcast a series of interviews with top thought leaders, to learn their thoughts and reactions to data presented across oncology during the conference.

Bevacizumab combined with first-line chemotherapy was shown to improve progression-free survival and overall survival in patients with advanced ovarian clear cell carcinoma.

Pembrolizumab plus chemotherapy with or without bevacizumab prolonged progression-free survival and overall survival in key subgroups of patients with persistent, recurrent, or metastatic cervical cancer, displaying benefits similar to those seen in the broader patient population.

Twice-daily oral ruxolitinib plus carboplatin and paclitaxel given as frontline neoadjuvant and post-surgical treatment to patients with stage III or IV ovarian cancer was found to be feasible and to improve progression-free survival compared with chemotherapy alone.

The combination of lenvatinib and pembrolizumab demonstrated clinically meaningful improvements in progression-free survival on next line of therapy among all-comer patients with advanced endometrial cancer and those with DNA mismatch repair proficient disease, according to an exploratory analysis of the phase 3 KEYNOTE-775 trial.

For the 10th consecutive year, OncLive® is honored to recognize oncology leaders whose innovations have contributed to immeasurable improvements in outcomes for countless patients.

Galinpepimut-S in combination with the PD-1 inhibitor pembrolizumab elicited a clinical benefit in patients with Wilms’ tumor-1-positive relapsed/refractory platinum-resistant advanced metastatic ovarian cancer, according to top line-line data from a phase 1/2 trial (NCT03761914).