Lung Cancer
Latest News

Latest Videos

CME Content
More News

The pathologic analysis of lung cancer has become increasingly complex, calling for continued attention to improving the accuracy of the assessment and reducing the amount of tissue required.

The FDA has approved the next-generation ALK inhibitor ceritinib as a treatment for ALK-positive patients with metastatic non-small cell lung cancer following treatment with crizotinib.

Balazs Halmos, MD, section chief of Thoracic Oncology at New York-Presbyterian Hospital/Columbia University Medical Center, discusses the impact of EGFR inhibitors on the treatment of lung cancer.
The treatment decisions for NSCLC are primarily dependent on the patient's performance status, extent of disease, and histological subtype. Significant developments in the area of targeted therapies have changed the treatment paradigm for NSCLC.

Locally advanced non-small cell lung cancer (NSCLC) remains a challenging disease to treat, with a 5-year survival rate for patients with unresectable stage III disease of approximately 20%, even after definitive radiation therapy and concurrent chemotherapy.

Every patient with a pancoast tumor of the lung should be evaluated by a Pancoast-experienced thoracic surgeon (and neurosurgeon) before ruling out surgery, and before starting induction therapy.

PD-L1 levels adequately predict response and clinical outcomes for PD-1 inhibitor MK-3475 in patients with non-small cell lung cancer (NSCLC) and melanoma

In more than 40 years as a National Cancer Institute (NCI)-designated cancer center-and the first center in the network devoted purely to basic research-The Wistar Institute has built a reputation for furthering the sort of scientific research that will improve clinical cancer medicine.

Balazs Halmos, MD, section chief of Thoracic Oncology at New York-Presbyterian Hospital/Columbia University Medical Center, discusses the anti-PD-L1 antibody MK-3475 and the challenge of integrating immunotherapies into the field of lung cancer.

The large phase III MAGRIT study investigating the MAGE-A3-specific vaccine GSK1572932A for patients with non-small cell lung cancer (NSCLC) will be completely halted following an interim analysis that demonstrated a lack of benefit.

The next-generation EGFR inhibitor CO-1686 has demonstrated promising activity in T790M-positive patients with non-small cell lung cancer.

Ceritinib (LDK378) demonstrated an ORR of 58% and a median PFS of 7 months as a treatment for patients with ALK-positive non-small cell lung cancer.

Balazs Halmos, MD, section chief of Thoracic Oncology at NewYork-Presbyterian Hospital/Columbia University Medical Center, discusses the implication of the IPASS study.

The MAGE-A3-specific immunotherapeutic GSK1572932A failed to significantly extend disease-free survival (DFS) in patients with resected nonmetastatic non-small cell lung cancer (NSCLC) who tested negative for a specific gene expression signature
Thomas J. Lynch, MD, Richard Sackler and Jonathan Sackler Professor of Medicine (Medical Oncology), director, Yale Cancer Center, physician-in-chief, Smilow Cancer Hospital at Yale-New Haven, Giant of Lung Cancer Care, discusses advances in molecular profiling in lung cancer.
Bavituximab, Peregrine Pharmaceuticals' lead clinical immunotherapeutic candidate, received Fast Track designation from the FDA to kick off 2014.
A phase III study exploring the MET inhibitor onartuzumab as a treatment for patients with NSCLC is being stopped, following an interim analysis that suggested a lack of clinically meaningful efficacy.
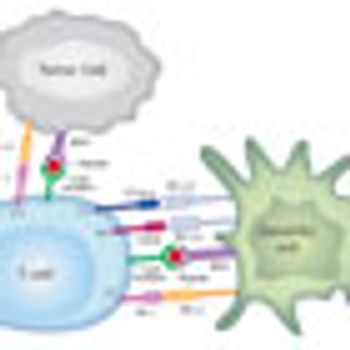
Inhibitory receptors such as anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) expressed on tumor-specific T cells lead to compromised activation and suppressed effector functions such as proliferation, cytokine secretion, and tumor cell lysis.

The second-line administration of ramucirumab in combination with docetaxel has shown a statistically significant improvement in overall survival and progression-free survival compared with placebo plus docetaxel in patients with non-small cell lung cancer
Roy S. Herbst, MD, PhD, a professor of medicine at the Yale Cancer Center and chief of medical oncology at Smilow Cancer Hospital at Yale-New Haven in Connecticut, discusses the using immunotherapy agents to treat patients with lung cancer.
The members of the RAS oncogene family are central cogs in many different cell-signaling pathways, coordinate a variety of important cellular processes, and are highly mutated in a number of different cancers, including several with extremely poor prognosis.

Alice T. Shaw, MD, PhD, discusses the two most advanced next-generation ALK-inhibitors for the treatment of non-small cell lung cancer (NSCLC): LDK378 and alectinib (AF802).