
Urothelial Cancer
Latest News

Treatment Sequencing in Refractory Urothelial Carcinoma Requires a Personalized Approach

FDA Approval Sought for Erdafitinib in Locally Advanced or Metastatic FGFR3+ Urothelial Carcinoma
Latest Videos

CME Content
More News

Guru P. Sonpavde, MD, discusses the importance of utilizing precision medicine in bladder cancer, and highlights the rationales and designs of the ongoing phase 3 EV-302 and AMBASSADOR trials.

Shilpa Gupta, MD, expands on the updated results from EV-103 in patients with locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin, and details the next steps for investigating the combination of enfortumab vedotin plus pembrolizumab in the ongoing phase 3 EV-302 trial.

Petros Grivas, MD, PhD, discusses several unmet needs within urothelial cancer, as well as how to potentially ameliorate them.

Guru P. Sonpavde, MD, discusses the future of bladder cancer treatment, including potential novel targets and further research with immunotherapy and antibody-drug conjugates.

Roger Li, MD, expands on the mechanism of action for the combination of CG0070 and pembrolizumab, and discusses the rationale for investigating the regimen's synergy in patients with non–muscle invasive bladder cancer who are unresponsive to Bacillus Calmette-Guérin.

Guru P. Sonpavde, MD, discusses key data presented from the phase 3 THOR and SWOG S1011 studies at the 2023 ASCO Annual Meeting, and details ongoing trials that could help advance precision medicine approaches for patients with bladder cancer.
Patients with low-grade upper tract urothelial carcinoma experienced similar rendered disease free rates, regardless of surgery type or tumor volume, following treatment with mitomycin for pyelocalyceal solution.

Guru P. Sonpavde, MD, discusses results from the phase 3 SWOG S1011 trial conducted in patients with muscle-invasive bladder cancer.

Treatment with UGN-102 with or without transurethral resection of bladder tumors led to an improvement in disease-free survival compared with TURBT alone in patients with low-grade, intermediate-risk non–muscle-invasive bladder cancer.

Matthew Galsky, MD, discusses how updated findings from the phase 3 CheckMate 274 study confirm the efficacy of adjuvant systemic immunotherapy in metastatic urothelial carcinoma.

Shilpa Gupta, MD, discusses the enrollment criteria used for the phase 1/2 EV-103 trial in patients with locally advanced or metastatic urothelial carcinoma, and highlights next steps for the investigation of the frontline combination of enfortumab vedotin and pembrolizumab in the phase 3 EV-302 trial.

Responses achieved with enfortumab vedotin plus pembrolizumab were found to be rapid and durable in patients with previously untreated locally advanced or metastatic urothelial carcinoma who were cisplatin ineligible.

Frontline treatment with the combination of nivolumab and cisplatin-based chemotherapy, followed by nivolumab monotherapy, led to a statistically significant improvement in PFS and OS vs standard cisplatin-based chemotherapy regimens alone in previously untreated, cisplatin-eligible patients with unresectable or metastatic urothelial carcinoma.

Matthew Galsky, MD, discusses final results from the phase 3 IMvigor130 trial in patients with metastatic urothelial cancer.

Single-agent enfortumab vedotin in the neoadjuvant setting had substantial antitumor activity and a high event-free survival rate with a manageable toxicity profile in patients with cisplatin-ineligible muscle-invasive bladder cancer.

The combination of disitamab vedotin with toripalimab generated responses and displayed a manageable safety profile in patients with locally advanced or metastatic urothelial carcinoma, irrespective of HER2 expression.

The addition of first-line maintenance avelumab to best supportive care has sustained tolerability in patients with advanced urothelial carcinoma.

Shilpa Gupta, MD, discusses the design, methodology, and potential significance of the ongoing phase 3 MAIN-CAV trial in patients with locally advanced/metastatic urothelial carcinoma

Shilpa Gupta, MD, discusses the 4-year safety and efficacy data derived from the phase 1/2 EV-103 trial in patients with locally advanced or metastatic urothelial carcinoma.
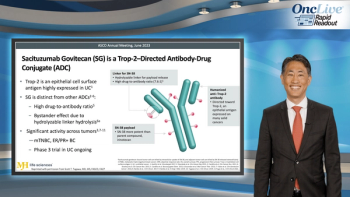
Expert Scott Tagawa, MD, MS, FASCO, FACP, reviews recent clinical trial data on UGT1A1- and Trop-2–guided treatment of metastatic urothelial carcinoma.

Extended lymphadenectomy at the time of radical cystectomy did not produce a significant improvement in disease-free survival (DFS) or overall survival (OS) vs standard lymphadenectomy for patients with muscle-invasive bladder cancer.

The combination of enfortumab vedotin-ejfv and pembrolizumab generated rapid and durable responses and demonstrated a manageable safety profile in patients with locally advanced or metastatic urothelial carcinoma who were ineligible for cisplatin.
Erdafitinib reduced the risk of death by 36% vs investigator’s choice of chemotherapy in patients with FGFR2/3-altered metastatic urothelial cancer who were previously treated with anti–PD-1 therapy, according to findings from the phase 3 THOR trial.

Shilpa Gupta, MD, discusses the real-world benefit that may be derived based on findings from the long-term analysis of the phase 3 JAVELIN Bladder 100 trial in patients with advanced urothelial carcinoma.

Andrew Katims, MD, MPH, discusses the feasibility of using single-cell RNA-sequencing in FGFR3-mutated upper tract urothelial carcinoma











































