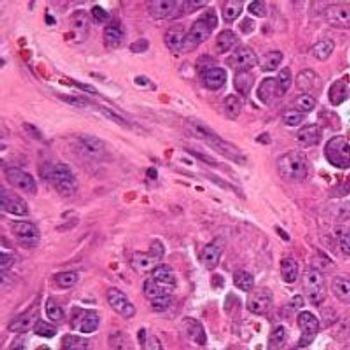
In a 16 to 0 vote, the FDA’s Oncologic Drugs Advisory Committee voted that sufficient evidence has not been provided to conclude that 131I-omburtamab improves overall survival for pediatric patients with central nervous system /leptomeningeal metastases from neuroblastoma.