Breast Cancer
Latest News

Latest Videos

CME Content
More News

A biomarker analysis of participants in a phase II breast cancer trial demonstrated potential for identifying tumor markers to predict susceptibility to specific therapies.

The HER2-enriched subtype of HER2-positive breast cancer is a strong predictor of sensitivity to dual HER2 blockade without the use of chemotherapy.

Adjuvant ibandronate (Boniva) added to hormone therapy did not provide a clinical benefit to postmenopausal patients with HR-positive, early-stage breast cancer, according to findings from the phase III TEAM IIB trial.

A neoadjuvant regimen combining the CDK4/6 inhibitor abemaciclib with anastrozole induced a response rate of 54.7% in patients with HR+/HER2-negative early-stage breast cancer, according to findings from the phase II neoMONARCH trial.

The addition of an aromatase inhibitor (AI) to pertuzumab and trastuzumab improved progression-free survival by 3.09 months, when compared with trastuzumab plus an AI.

Median progression-free survival was improved by 2.1 months with the addition of the pan-PI3K inhibitor buparlisib to fulvestrant for women with HR-positive/HER2-negative advanced breast cancer who received a prior aromatase inhibitor and progressed on or after an mTOR inhibitor.

First results from the phase III DATA study show no advantage to extending anastrozole therapy from 3 years to 6 for the primary endpoint of 5-year adapted disease-free survival.

Extending letrozole therapy in women with early-stage, HR-positive breast cancer who completed 5 years of prior hormone therapy did not yield a statistically significant improvement in either disease-free or overall survival, but prolonged use of the aromatase inhibitor may be beneficial in some subgroups of women with a higher risk of recurrence.

The genomic landscape of recurrent metastatic estrogen receptor–positive breast cancer differed significantly from the mutational profile of primary disease in a study that sheds light on acquired resistance mechanisms to anticancer therapies.

Higher levels of tumor-infiltrating lymphocytes were associated with improvements in overall survival for patients with advanced HER2-positive breast cancer treated with docetaxel, trastuzumab, and pertuzumab in the phase III CLEOPATRA trial.

Adding the PARP inhibitor veliparib to carboplatin/paclitaxel chemotherapy induced a response rate of 77.8% in patients with advanced BRCA-positive breast cancer.
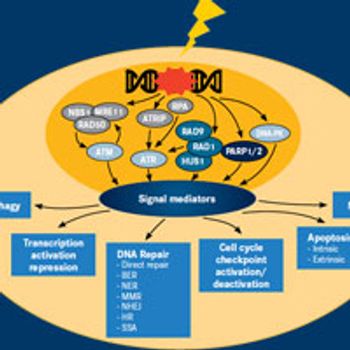
A coordinated network of signaling pathways works to protect the cell from the toxic effects of DNA damage.

Matthew J. Ellis, MB BChir, PhD, FRCP, provides his expertise on the genomics and molecular profiling of breast cancer.

Results from the phase III PALOMA-2 study—which demonstrated a significant progression-free survival advantage with palbociclib (Ibrance) plus letrozole compared with letrozole alone in ER-positive, HER2-negative metastatic breast cancer—were recently published in The New England Journal of Medicine.
Lisa Carey, MD, associate director, Clinical Research, UNC Lineberger Comprehensive Cancer Center, Richardson and Marilyn Jacobs Preyer Distinguished Professorship for Breast Cancer Research, UNC-Chapel Hill, discusses how she has seen various agents impact quality of life (QOL) when used to treat patients with breast cancer.

Paul Kelly Marcom, MD, associate professor of Medicine, Duke University School of Medicine, Duke Cancer Institute, discusses the impact that the FALCON trial findings have had on the field of hormone receptor-positive breast cancer.

There are a wide variety of novel agents currently being investigated in the neoadjuvant setting for patients with HER2-positive breast cancer.
Debu Tripathy, MD, chair of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, discusses the potential role of immunotherapy in the treatment of patients with HER2-positive breast cancer.

Patrick Borgen, MD, chair, Department of Surgery, director, Breast Center, Maimonides Medical Center, discusses the successful progress seen in the treatment landscape of HER2-positive breast cancer.

Immunotherapy and chemotherapy are options physicians should not dismiss for patients with HER2-positive breast cancer, according to Lisa Carey, MD, even with the available targeted agents trastuzumab, pertuzumab, lapatinib, and ado-trastuzumab emtansine.
Debu Tripathy, MD, chair of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, discusses agents that are available and in development in the neoadjuvant setting as treatments for patients with HER2-positive breast cancer.

Dual HER2-directed therapy has demonstrated promising efficacy as a treatment approach for patients with breast cancer. Still, there remains a need for further research pertaining to therapies for HER2-positive patients who do do not meet the current criteria for this neoadjuvant regimen.

Findings from a highly anticipated, randomized, phase II trial could possibly pave the path for the FDA approval of the first targeted therapy for patients with triple-negative breast cancer, explains Linda T. Vahdat, MD.
Carey Anders, MD, assistant professor for the Department of Medicine, Division of Hematology and Oncology, at UNC-Chapel Hill, UNC Linebarger Comprehensive Cancer Center, discusses systemic agents available and in development for patients with breast cancer who also have brain metastases.

Richard Finn, MD associate professor of Medicine at the UCLA David Geffen School of Medicine, discusses what’s next for palbociclib following the PALOMA trials.