
Oncology Business Management
Latest News

Latest Videos

CME Content
More News

The ACCC David King Community Clinical Scientist Award was awarded to Paul D. Hansen, MD, FACS, a pioneering leader in research on minimally invasive approaches to major liver and pancreas surgery and the promise these techniques hold for improving the quality of care, the patient experience, and potentially reducing healthcare costs.

With just 2 more years to go in the Oncology Care Model, CMS is proposing a next-generation payment system that would include more private payers and hold practices more accountable for quality and costs of care.

President Donald Trump is nominating Stephen M. Hahn, MD, FASTRO, as the next commissioner of the FDA.

In July 2019, Centers for Medicare & Medicaid proposed the Radiation Oncology Model, an important step forward in allowing the nation’s 4500 radiation oncologists to join in the transition to value-based healthcare.

Studies have raised doubts about the value Medicare and other payers are getting for the care they pay for, including in oncology. This has intensified the demand for new value-based care models such as the Merit-based Incentive Payment System and the Oncology Care Model.

Certain themes and questions about individualized, scientifically unanswerable dilemmas often recur when talking with patients and families about treatment.

In an interview with OncologyLive®, Scott Gottlieb, MD, shared his insights on developments in the oncology arena.

The artificial intelligence system that IBM designed for oncology has demonstrated clinical utility in recent studies, generating excitement that the technology may have a role as a decision support tool.

A partnership between a healthcare system in Morristown, New Jersey, and a genomics research institute in Phoenix, Arizona, has led to the opening of a center dedicated to offering phase I cancer trials in community settings and improving access for patients with limited coverage.

Even if a disease is diagnosed as “incurable,” or progression to a state of incurability subsequently develops, the time between diagnosis and death is being prolonged and the quality of life improved with novel oncologic interventions, such that continued therapeutic efforts are justified.

With the recent launch of a free, open-source system for standardizing the electronic storage of patient records, the American Society of Clinical Oncology hopes to improve information flow across electronic health record platforms and curtail the harms of information blocking by private aggregators and vendors of patient information.

Co-chairs William K. Oh, MD, Adam M. Brufsky, MD, PhD, and Benjamin P. Levy, MD, preview key topics that will be covered at the 37th Annual CFS®: Innovative Cancer Therapy for Tomorrow symposium.

There is no more important principle in the conduct of legitimate therapeutic investigation than ensuring the adequacy of informed consent of the prospective clinical trial participant.

Atlantic Health System of Morristown New Jersey and Translational Genomics Research Institute of Arizona have opened a center dedicated to phase I clinical trials in cancer and improving access for patients with limited coverage.
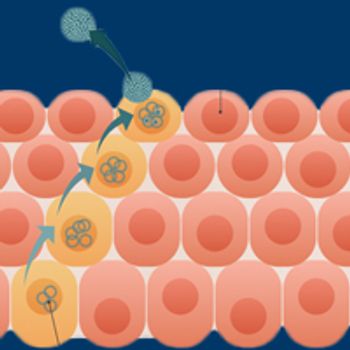
The development of therapeutic vaccines for patients with cancers associated with the human papillomavirus has emerged as a leading strategy in continuing research efforts to address the growing public health threat posed by the virus.

With the revolution in our understanding of cancer’s basic molecular biology, it is increasingly evident that subgroups of cancer originating from specific regions of the body have unique natural histories and respond to very different therapeutics. For example, the importance of BRCA mutations, which define a subset of ovarian cancers impressively sensitive to PARP inhibitors, has striking altered the management of this group of gynecologic malignancies.

Given the widespread financial impact the high cost of cancer drugs has on the healthcare system, it may be time for the FDA to take a broader approach when reviewing oncology drug applications. By also examining the cost of the drug, the FDA could shine a light on escalating drug costs and play a leadership role in creating new programs and initiatives that help patients pay for promising new therapies.
OncLive interviewed experts at the State of the Science Summits in August 2019 on aspects of care in their field that they wish were publicized more.

Kristin Anderson, PhD, shares her journey from patient to investigator.

Research forms the backbone of medical knowledge, and research integrity is a crucial responsibility of any scientist.

Yara Abdou, MD, sheds light on the challenging path of becoming an oncologist.

The cost of antineoplastic therapy and essential supportive care medications have a substantial negative impact on patients with cancer and their families.

A 2018 Giants of Cancer Care award winner for Drug Development, Joseph R. Bertino, MD, was an early pioneer in developing the understanding of methotrexate and its administration in cancer. His work has served as a platform for much broader investigation into the optimal use of other cancer agents. Along the way, Bertino has mentored many leaders in oncology and hematology and earned international recognition.

Residents of Indiana now have a National Cancer Institute–designated Comprehensive Cancer Center in their state, and Floridians have another designated Cancer Center in theirs. Both institutions, the Indiana University Melvin and Bren Simon Cancer Center in Indianapolis and the University of Miami Sylvester Comprehensive Cancer Center, are OncLive® Strategic Alliance Partners.











































