Genitourinary Cancers
Latest News
Latest Videos

CME Content
More News
Ignacio Duran, MD, PhD, reflects on the JAVELIN Bladder 100 trial, which analyzed avelumab as frontline maintenance therapy in metastatic urothelial carcinoma.

Considerations for the role of immunotherapy in metastatic urothelial carcinoma as maintenance or switch therapy.

Expert perspective on the current treatment paradigm of metastatic urothelial carcinoma across different regions of the world.

The combination of nivolumab and cabozantinib was associated with improved quality of life compared with sunitinib in patients with previously untreated advanced renal cell carcinoma, according to patient-reported outcome data from the phase 3 CheckMate-9ER trial.

The FGFR TKI erdafitinib continued to provide consistent clinical benefits with a manageable safety profile when used in the second-line treatment of patients with locally advanced or metastatic urothelial carcinoma harboring FGFR alterations.

Matthew Galsky, MD, discusses clinical trials that addressed key questions in urothelial cancer.

Over a 42-month period, the combination of nivolumab and ipilimumab induced a longer treatment-free survival compared with sunitinib (Sutent) when used as frontline therapy in patients with advanced renal cell carcinoma.

Ziad Bakouny, MD, MSc, discusses understanding the biology of translocation renal cell carcinoma.

Sarah Elizabeth Yentz, MD, discusses translating data to real-world clinical practice in renal cell carcinoma.
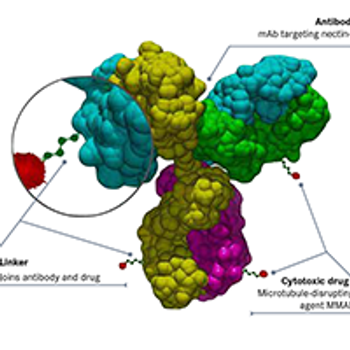
Most ongoing clinical trials exploring nectin-4 as a target involve studies of enfortumab vedotin and its potential synergy with immune checkpoint inhibitors in bladder cancer, while several other early-phase studies are testing novel agents in solid tumors.

Matthew Galsky, MD, discusses the evolution of standard of care treatment options in urothelial carcinoma.

A new drug application has been submitted to the FDA seeking the marketing approval of dovitinib as a potential option in the third-line treatment of patients with renal cell carcinoma.

The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended the approval of single-agent pembrolizumab for use as an adjuvant treatment in adult patients with renal cell carcinoma who are at increased risk of recurrence after nephrectomy, or following nephrectomy and the resection of metastatic lesions.

The European Medicines Agency’s Committee for Medicinal Products for Human Use has adopted a positive opinion in favor of the approval of enfortumab vedotin as a monotherapy in the treatment of adult patients with locally advanced or metastatic urothelial cancer.

Pembrolizumab demonstrated a statistically significant and clinically meaningful improvement in disease-free survival vs placebo as a postnephrectomy adjuvant therapy in patients with renal cell carcinoma at intermediate-high or high risk of recurrence, and in those who are M1 with no evidence of disease.

Dr. Choueiri discusses the significance of the FDA approval of pembrolizumab as adjuvant therapy in renal cell carcinoma and key data from the pivotal phase 3 KEYNOTE-564 trial.
The combination of pembrolizumab and axitinib demonstrated comparable activity and safety vs sunitinib in Japanese patients vs the global population of patients with newly diagnosed metastatic renal cell carcinoma enrolled in the phase 3 KEYNOTE-426 trial.

Manojkumar Bupathi, MD, MS, shares the main highlights from the meeting, which centered on frontline, second, and later-line treatment of metastatic urothelial carcinoma and metastatic renal cell carcinoma.

Toni K. Choueiri, MD, discusses the FDA approval of adjuvant pembrolizumab in renal cell carcinoma.

Caroline Nebhan, MD, PhD, discusses the efficacy of checkpoint inhibitors in older patients with cancer.

Brian Ramnaraign, MD, discusses current first-line treatment guidelines for patients with metastatic renal cell carcinoma.

The FDA has approved pembrolizumab for the adjuvant treatment of patients with renal cell carcinoma who are at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.

For patients with advanced clear cell renal cell carcinoma, it will be of utmost importance to recognize the variation in response and type of progression with first-line therapy, the patient’s characteristics and motivations, and the promise of new agents and combinations.

Manojkumar Bupathi, MD, MS, discusses the improvement in first-line treatment options for patients with clear cell renal cell carcinoma.

The COVID-19 vaccine was safe and well tolerated in patients who received immune checkpoint inhibitors for renal cell carcinoma or melanoma.