Immuno-Oncology
Latest News
Latest Videos

CME Content
More News

Balazs Halmos, MD, section chief of Thoracic Oncology at New York-Presbyterian Hospital/Columbia University Medical Center, discusses the anti-PD-L1 antibody MK-3475 and the challenge of integrating immunotherapies into the field of lung cancer.
The large phase III MAGRIT study investigating the MAGE-A3-specific vaccine GSK1572932A for patients with non-small cell lung cancer (NSCLC) will be completely halted following an interim analysis that demonstrated a lack of benefit.

Oliver Sartor, MD, board professor, cancer research, Tulane University, discusses a trial that would study Provenge (sipuleucel-T) in combination with radium-223 as a potential treatment option for patients with prostate cancer.
Renier J. Brentjens, MD, PhD, associate professor, chief, Cellular Therapeutics Center, Memorial Sloan Kettering Cancer Center, discusses the challenges associated with CAR-modified T cells.

Robert Dreicer, MD, MS, discusses combining and sequencing agents with immunotherapies for the treatment of prostate cancer.

The MAGE-A3-specific immunotherapeutic GSK1572932A failed to significantly extend disease-free survival (DFS) in patients with resected nonmetastatic non-small cell lung cancer (NSCLC) who tested negative for a specific gene expression signature

Increasing efficacy with immunotherapies in some cancers led to the strategy being deemed Breakthrough of the Year by Science magazine in 2013

The oncolytic immunotherapeutic vaccine talimogene laherparepvec (T-VEC) promoted tumor shrinkage in 64% of patients with advanced melanoma, including a marked reduction in the size of uninjected metastatic lesions

Nivolumab, a PD-1-specific antibody, has been shown to produce long-term remissions with limited toxicity in patients with advanced melanoma, according to results from one of the longest follow ups to examine the drug.

Karl Lewis, MD, medical oncologist, associate professor, University of Colorado, discusses sequencing strategies for the treatment of melanoma.

Although breakthrough successes are generating a renaissance for anticancer immunotherapies, the framework for translating promising research results into clinical practice remains very much under construction.

Since its approval in 1998 to treat metastatic breast cancer, the anti-HER2 monoclonal antibody trastuzumab has dramatically expanded life expectancy and improved quality of life for women diagnosed with HER2-positive disease.
Bavituximab, Peregrine Pharmaceuticals' lead clinical immunotherapeutic candidate, received Fast Track designation from the FDA to kick off 2014.
An immunotherapy drug for the treatment of prostate cancer, approved in the US in 2010, will soon be available in Europe.

David F. McDermott, MD, associate professor, Department of Medicine, Harvard Medical School/Dana-Farber Cancer Institute, discusses the potential of targeting PD-1 or PD-L1 in patients with kidney cancer
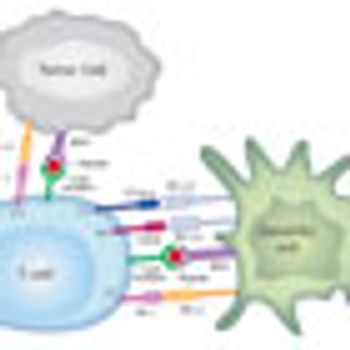
Inhibitory receptors such as anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) expressed on tumor-specific T cells lead to compromised activation and suppressed effector functions such as proliferation, cytokine secretion, and tumor cell lysis.

A personalized vaccine being tested as a therapy for recurrent glioblastoma multiforme (GBM) improved patient survival compared with standard treatments
Roy S. Herbst, MD, PhD, a professor of medicine at the Yale Cancer Center and chief of medical oncology at Smilow Cancer Hospital at Yale-New Haven in Connecticut, discusses the using immunotherapy agents to treat patients with lung cancer.

Merck announced the signing of three separate clinical collaboration agreements to evaluate the potential of its investigational anti-PD-1 immunotherapy MK-3475 across multiple tumor types

High tumor expression of the protein PD-L1 is independently associated with shorter OS in patients with mRCC receiving treatment with VEGF-targeted therapy.

Robert Figlin, MD, FACP, discusses updated data on the ADAPT trial, which is an ongoing international phase III randomized trial of autologous dendritic cell immunotherapy (AGS-003) plus standard treatment in metastatic renal cell carcinoma (mRCC)

Leonard Gomella, MD, from the Kimmel Cancer Center Network, Thomas Jefferson University Hospital, provides an update on the dendritic cell cancer vaccine sipuleucel-T in castration-resistant prostate cancer.
Immunotherapy has become an increasingly appealing therapeutic strategy for patients with cancer, with many late-stage clinical trials demonstrating overall survival (OS) advantages in melanoma and castrationresistant prostate cancer.

Harnessing multiple components of the immune system to fight cancer is the focus of NewLink Genetics' clinical pipeline.

The anti-PD-1 monoclonal antibody pidilizumab showed promising clinical activity and was safely administered to patients with diffuse large B-cell lymphoma after autologous hematopoietic stem-cell transplantation.