
Sarcomas
Latest News

Latest Videos

CME Content
More News

R. Lor Randall, MD, FACS, discusses the standard treatment approach for patients with diffuse-type tenosynovial giant cell tumor, the results of the retrospective cohort study evaluating 1- vs 2-stage synovectomies in these patients, and the importance of having additional treatment options for this patient population.

A full dose of lurbinectedin plus a low dose of doxorubicin was clinically active and tolerable in patients with advanced or metastatic soft tissue sarcomas, supporting its continued investigation in those with leiomyosarcoma.

The FDA has extended the Prescription Drug User Fee Act decision date by 3 months to allow more time to complete their review of the new drug application seeking the approval of nirogacestat in the treatment of adult patients with desmoid tumors.

Treatment with doxorubicin plus zalifrelimab and balstilimab produced a favorable 6-month progression-free survival rate in patients with difficult-to-treat soft tissue sarcoma subtypes unlikely to respond to doxorubicin or immune checkpoint inhibitor monotherapy.

Treatment with the investigational gamma secretase inhibitor nirogacestat allowed patients with progressing desmoid tumors to experience a significant and clinically meaningful reduction in several aspects of disease-related pain when compared with placebo.

The China National Medical Products Adminstration’s Center for Drug Evaluation has recommended that olverembatinib receive breakthrough therapy designation for the treatment of patients with gastrointestinal stromal tumor that is succinate dehydrogenase deficient.
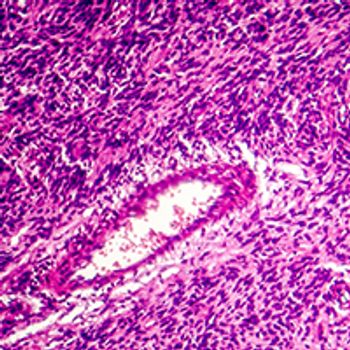
Treatment with milademetan did not lead to a statistically significant improvement in progression-free survival compared with trabectedin in patients with dedifferentiated liposarcoma, failing to reach the primary end point of the phase 3 MANTRA trial.

The FDA has placed a partial clinical hold on the phase 1 trial evaluating the safety, tolerability, pharmacokinetics, pharmacodynamics, and early clinical activity of FHD-609 in patients with advanced synovial sarcoma and SMARCB1-deleted tumors.

The combination of PARP and ATR inhibition with olaparib and ceralasertib was tolerable but showed limited efficacy in pediatric patients with advanced malignancies harboring DNA replication stress and DNA repair deficiencies, according to findings from arm N of the phase 1/2 AcSé-ESMART trial.

Thrombocytopenia is a common adverse effect associated with treatment with the MDM2 inhibitor milademetan in patients with sarcomas and other solid tumors, and the effects of thrombocytopenia can be mitigated with an intermittent dosing schedule and managed with dose interruptions or reductions.

The FDA has granted an orphan drug designation to TP-1287, an investigational oral CDK9 inhibitor, for the treatment of patients with Ewing sarcoma.

The oral MDM2/p53 antagonist BI 907828 elicited preliminary antitumor activity and had a manageable safety profile in patients with MDM2-amplified dedifferentiated liposarcoma.

R. Lor Randall, MD, FACS, expands on data from a phase 3 trial evaluating the addition of ganitumab to standard-of-care, interval-compressed chemotherapy, the implications of these results, and how the study could help inform future trials exploring other targeted therapies for the treatment of patients with metastatic Ewing sarcoma.

R. Lor Randall, MD, FACS, discusses the potential symptoms and presentation of mesenchymal chondrosarcoma, as well as highlighted recent case studies that he consulted on for patients with mesenchymal chondrosarcoma.

R. Lor Randall, MD, FACS, discusses, the importance of correctly diagnosing mesenchymal chondrosarcoma.

R. Lor Randall, MD, FACS, discusses the presentation and diagnosis of mesenchymal chondrosarcomas, the role of HEY1-NCoA2 gene fusions in the disease, and potential treatment options for this rare subgroup of patients.

R. Lor Randall, MD, FACS, discusses how sarcoma experts from around the world connected to collaborate on the ISKS, the importance of identifying and better understanding sarcoma subgroups, and how these findings could inform prescreening for those at risk for the disease.

The FDA has granted a priority review to the new drug application for nirogacestat for the treatment of adult patients with desmoid tumors.

Dr D’Amato discusses research in sarcoma and gastrointestinal stromal tumor, incorporating circulating tumor DNA analysis into routine reevaluation of patients with progressive disease, and the importance of establishing a drug’s clinical profile in non–trial target ethnic populations.

The selective small molecule MDM2 inhibitor milademetan administered at an intermittent dosing schedule was tolerable and showed early efficacy in patients with advanced cancers, according to findings from a phase 1 study.

The FDA has accepted for review a biologics license application for a proposed denosumab biosimilar, according to an announcement from Sandoz, Inc.

The FDA has granted a breakthrough therapy designation to pimicotinib for the treatment of patients with tenosynovial giant cell tumor who are not amenable for surgery.

R. Lor Randall, MD, FACS, discusses the outcomes of combining surgery and pexidartinib in patients with tenosynovial giant cell tumors, what investigators still need to learn about sequencing the CSF1R inhibitor around surgery, and what the combination approach could mean for patients with TGCT.
INT230-6 demonstrated direct tumor killing in soft tissue sarcoma and elicits an anti-cancer immune response within the injected tumor both alone and in combination with ipilimumab.

Dr Somaiah discusses the excitement surrounding novel agents such as CSF1R inhibitors and ULK1/2 inhibitors, and the utility of broad spectrum TKIs such as ripretinib.












































