CAR T-cell Therapy
Latest News

Latest Videos

CME Content
More News

December 5, 2020 - The enriched chimeric antigen receptor T-cell therapy bb21217 improved responses and also prolonged duration of response compared with non-enriched CAR T cells in patients with relapsed/refractory multiple myeloma.

Nilanjan Ghosh, MD, PhD, director of the Lymphoma Program and a physician with Levine Cancer Institute, discusses unmet needs with CAR T-cell therapy in diffuse large B-cell lymphoma.
Joshua Brody, MD, discusses management strategies for the toxicities associated with CAR T-cell therapy in mantle cell lymphoma.

Stephen A. Strickland, MD, BS, MS, MSCI, relays insightful questions from the audience to the speakers, who provided insight into how they are navigating the evolving treatment landscape of hematologic malignancies.

Olalekan O. Oluwole, MBBS, MD, discusses the approvals of tisagenlecleucel and axicabtagene ciloleucel, along with several research efforts that have been dedicated to the development and exploration of CAR T-cell therapies in the realm of leukemias and lymphomas.

November 18, 2020 - The FDA has decided to lift the clinical hold placed on the phase 1 MELANI-01 trial, which is examining the CAR T-cell therapy UCARTCS1 as a treatment for patients with relapsed/refractory multiple myeloma.

November 17, 2020 - The review of the biologics license application for the CAR T-cell product lisocabtagene maraleucel for the treatment of adult patients with relapsed/refractory large B-cell lymphoma following at least 2 previous therapies has been delayed.
Hundreds of trials are under way, 3 CAR T-cell therapies for hematologic malignancies are on the market, and 2 new products may receive FDA approval in the next several months, including a BCMA–directed therapy that is poised to help transform treatment of multiple myeloma.

The emergence of cellular-based therapies represents a major opportunity to improve outcomes in the heavily pretreated and refractory myeloma population.


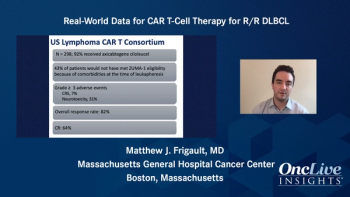


Brian T. Hill, MD, PhD, discusses the approval of brexucabtagene autoleucel in mantle cell lymphoma.

Ira Braunschweig, MD, discusses factors to consider when selecting between autologous stem cell transplant, allogeneic stem cell transplant, and CAR T-cell therapy in diffuse large B-cell lymphoma.

Faiz Anwer, MD, discusses the growing role of CAR T-cell therapy in multiple myeloma and areas of unmet need.

Abhinav Deol, MD, highlights the progress that has been made with CAR T-cell therapy in leukemia and lymphoma, challenges faced with regard to accessibility and toxicity, and next steps for this modality.
Caron Jacobson, MD, discusses the significance of CD19-targeted CAR T-cell therapy in B-cell malignancies.

Jeffrey Zonder, MD, discusses the explosion of targeted therapies, along with the rise of cellular therapy, in hematologic malignancies.

The investigational wholly-owned allogeneic CAR T-cell therapy CTX110 demonstrated dose-dependent efficacy and responses in patients with relapsed/refractory CD19-positive B-cell malignancies.
Brian T. Hill, MD, PhD, discusses the FDA approval of brexucabtagene autoleucel for patients with relapsed/refractory mantle cell lymphoma.

Brian T. Hill, MD, PhD, discusses the advent of CAR T-cell therapies, such as axicabtagene ciloleucel, tisagenlecleucel, and brexucabtagene autoleucel, and how they have shifted lymphoma treatment into a new era.