CAR T-cell Therapy
Latest News

P-PSMA-101 Elicits Encouraging Responses in Metastatic Castration-Resistant Prostate Cancer
Latest Videos

CME Content
More News

Due to the potency of CAR T-cell therapies and the associated adverse events that can to arise in patients receiving this type of treatment, oncology nurses need to be educated on how to best identify and manage these AEs.

Stephen M. Ansell, MD, PhD, discusses recent progress made with non–CAR T-based approaches in the non-Hodgkin lymphoma treatment paradigm, opportunities for sequencing available options, and ongoing efforts that are picking up momentum.

As more CAR T-cell therapies are approved for the treatment of patients with hematologic malignancies, adopting management strategies for cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome using newly standardized grading criteria is an important component of utilizing these products in practice.

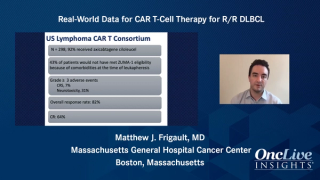
CAR T-cell therapy has proven to be an effective treatment option in non-Hodgkin lymphoma subtypes, such as diffuse large B-cell lymphoma, and mantle cell lymphoma.

Jae Park, MD, discusses the factors to consider when selecting among the available CAR T-cell therapies for the treatment of patients with mantle cell lymphoma.

The combination of a CF33-CD19 oncolytic virus technology and CyCART-19, is under development for use as a potential therapeutic option for patients with solid tumors.

Jae H. Park, MD, discusses emerging CAR T-cell therapies in hematologic malignancies.
Dose-dependent engraftment of the investigative CAR T-cell therapy CYAD-211 was demonstrated in the first patient with relapsed/refractory multiple myeloma to receive this dose in the phase 1 IMMUNICY-1 trial, and no graft-versus-host disease has been reported to date.

Repetitive locoregional infusion of HER2-specific CAR T cells did not induce any dose-limiting toxicities.

Paolo Ghia, MD, PhD, discusses key findings from the phase 2 CAPTIVATE trial in chronic lymphocytic leukemia.

Adam Sperling, MD, PhD, discusses future research with CAR T-cell therapy in multiple myeloma.

Adam Sperling, MD, PhD, discusses managing CAR T-cell therapy–related toxicities in multiple myeloma.

The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended that idecabtagene vicleucel receive conditional marketing authorization for use in adult patients with relapsed and refractory multiple myeloma who have received at least 3 prior therapies, including an immunomodulatory agent, a proteasome inhibitor, and a CD38-targeted antibody, and have progressed on their last therapy.

City of Hope has entered into a licensing agreement with Imugene Ltd. for the patents covering a combination immunotherapy that enables CD19-directed CAR T-cell therapies to target and eradicate difficult-to-treat solid tumors.

Narendranath Epperla, MD, MS, discusses the efficacy of brexucabtagene autoleucel in patients with mantle cell lymphoma.

Henry Chi Hang Fung, MD, discusses how the durable response of axi-cel fills an unmet need for this patient population and the potentially exciting future of the therapy.

Lisocabtagene maraleucel demonstrated a highly significant improvement in event-free survival, complete response rate, and progression-free survival over standard of care in the second-line treatment of patients with relapsed/refractory large B-cell lymphoma, meeting primary and secondary end points of the phase 3 TRANSFORM trial.
C-CAR066 exhibited a favorable safety profile and promising efficacy in adult patients with relapsed/refractory B-cell non-Hodgkin lymphoma who failed with prior CD19 CAR T-cell therapy.

Ciltacabtagene autoleucel, an investigational BCMA-directed CAR-T therapy, sustained efficacy and durable responses in heavily pretreated patients with relapsed/refractory multiple myeloma.

The autologous CAR T-cell product CART-ddBCMA was found to elicit a 100% objective response rate in patients with relapsed/refractory multiple myeloma, with deep and durable responses noted in those with poor prognostic factors.

A single infusion of brexucabtagene autoleucel, a CAR T-cell therapy, demonstrated robust and durable responses in heavily pretreated patients with relapsed/refractory B-cell acute lymphoblastic leukemia.

The CAR T-cell therapy idecabtagene vicleucel continues to demonstrate improved survival among heavily pretreated patients with relapsed/refractory multiple myeloma.

Monalisa Ghosh, MD, discusses the role of off-the-shelf CAR T-cell therapy in patients with multiple myeloma.
Tmunity Therapeutics, a clinical-stage biotherapeutics company, has halted the development of its lead CAR T-cell product following the deaths of 2 patients who were enrolled to a trial investigating its use in solid tumors.

The FDA has granted priority review to the biologics license application for ciltacabtagene autoleucel for the treatment of patients with relapsed/refractory multiple myeloma.