Immuno-Oncology
Latest News

Latest Videos

CME Content
More News

In August 2016, the FDA approved pembrolizumab for patients with platinum-refractory squamous cell carcinoma of the head and neck. Not only was it the first immunotherapy approved for head and neck cancer (HNC), but it marked the first new drug approval for HNC in the United States in 20 years.

Two of the most noteworthy developments in the oncology field during 2016 were the continued expansion of checkpoint blockade immunotherapy agents into more cancer types and the federal government’s plans for funding and remaking the research paradigm.

Don M. Benson Jr, MD, PhD, discusses the clinical development of KIR-targeted therapies.
Igor Puzanov, MD, director, Early Phase Clinical Trials Program, chief of Melanoma, coleader, CCSG Experimental Therapeutics Program, professor of Oncology, Department of Medicine, Roswell Park Cancer Institute, discusses ways to manage patients with melanoma who may experience adverse events in response to treatment with immunotherapy.

Omid A. Hamid, MD, chief, Translational Research and Immunotherapy, director, Melanoma Therapeutics, The Angeles Clinic and Research Institute, discusses how to accurately select patients who are likely to have durable and long responses with single-agent immunotherapy versus combination treatment.
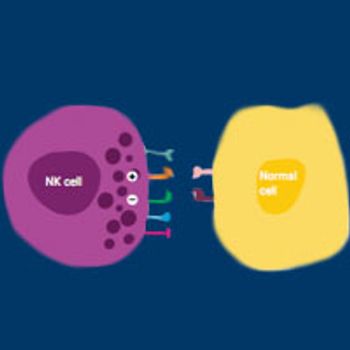
Although T cells have commanded most of the attention in the burgeoning immuno-oncology field, there is a growing appreciation that other immune cells have important roles in tumor surveillance and would represent attractive therapeutic targets.

Yvonne Saenger, MD, director of Melanoma Immunotherapy at Columbia University Medical Center, discusses immunotherapy combinations in melanoma.

The landscape of multiple myeloma continues to shift with more drug approvals, but pivotal results out of the 2016 ASH Annual Meeting will likely alter it even further.
Jedd D. Wolchok, MD, PhD, chief, Melanoma and Immunotherapeutics Service, Department of Medicine and Ludwig Center at Memorial Sloan Kettering Cancer Center, 2014 Giant of Cancer Care in Melanoma, discusses an ongoing clinical trial that will explore optimal treatments for patients with melanoma who fail on a PD-1 inhibitor as a single agent.

An immunotherapy combination that adds the interleukin-10 agonist AM0010 to FOLFOX chemotherapy will be evaluated in a phase III trial for patients with metastatic pancreatic cancer that could introduce a new modality to the treatment paradigm for the malignancy.

Naval Daver, MD, assistant professor, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, discusses research evaluating different immune checkpoint pathways in patients with acute myeloid leukemia.

Jason J. Luke, MD, an assistant professor of Medicine at the University of Chicago Medicine, discusses ongoing studies exploring immunotherapy for the treatment of patients with melanoma.
Everett Vokes, MD, John E. Ultmann Professor of Medicine and Radiation Oncology, physician-in-chief, University of Chicago Medical Center, chair, Department of Medicine, University of Chicago Medicine, discusses how to choose between nivolumab (Opdivo) and pembrolizumab (Keytruda) for second-line treatment for patients with head and neck cancer.

Negative reports evaluating molecularly selected agents should not derail the process of developing future therapies by employing a precision medicine approach.
Igor Puzanov, MD, director, Early Phase Clinical Trials Program, chief of Melanoma, coleader, CCSG Experimental Therapeutics Program, professor of Oncology, Department of Medicine, Roswell Park Cancer Institute, discusses an ongoing study exploring the optimal sequence of immunotherapy agents and targeted therapies for patients with melanoma.

Stefani Spranger, PhD, postdoctoral fellow, Cancer Research Institute at The University of Chicago Medicine, discusses how the presence or absence of CD8 T cells can affect treatment approaches for patients with melanoma.

Tanios Bekaii-Saab, MD, professor of Medicine, Mayo Clinic, discusses the role of immunotherapy in the treatment of patients with colorectal cancer.
Tanguy Y. Seiwert, MD, assistant professor of Medicine, University of Chicago Medicine, discusses the current prognosis for patients with head and neck cancer prior to the FDA approvals of immunotherapy agents nivolumab (Opdivo) and pembrolizumab (Keytruda) as treatments.
Jeffrey S. Weber, MD, PhD, deputy director and co-director of the Melanoma Program Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center, discusses the risks and benefits of combination immunotherapy as treatment for patients with melanoma.

Plasma- and urine-based assays designed to detect actionable mutations in patients with non–small cell lung cancer are changing the face of treatment for these patients.

Molecular testing is done for patients with non–small cell lung cancer to determine what genetic abnormalities are present. If a common-enough mutation is detected—such as EGFR or ALK—then patients are able to receive a targeted agent matched to that driver.
Stephen M. Ansell, MD, PhD, of Mayo Clinic, discusses studies exploring PD-1 inhibitors in Hodgkin lymphoma, specifically the CheckMate-039 trial.

Omid Hamid, MD, chief, Translational Research and Immunotherapy, director, Melanoma Therapeutics, The Angeles Clinic and Research Institute, discusses different immunotherapy approaches that oncologists are currently exploring in melanoma.