Melanoma & Skin Cancer
Latest News

Latest Videos

CME Content
More News
Isabella C. Glitza, MD, discusses a single-center phase I/Ib trial of concurrent intravenous and intrathecal nivolumab for patients with metastatic melanoma and leptomeningeal disease.
Grant McArthur, PhD, discusses significant results from the final analysis of the coBRIM trial, which evaluated the 5-year survival data of cobimetinib plus vemurafenib in patients with BRAF V600-mutated advanced melanoma.

Talimogene laherparepvec (T-VEC; Imlygic) prior to surgery was associated with improved recurrence-free survival and overall survival compared with surgery alone in patients with resectable advanced melanoma.

Ryan J. Sullivan, MD, discusses the significance of the BRAF/MEK combination dabrafenib and trametinib, which was the first BRAF/MEK inhibitor regimen to be approved by the FDA for the treatment of patients with BRAF V600E–positive stage III melanoma following complete resection.

Adil Daud, MD, discusses the role of dabrafenib plus trametinib in patients with advanced melanoma and highlighted other combinations under investigation.

Jeffrey S. Weber, MD, PhD, discusses the phase III EORTC1325/KEYNOTE-054 trial, which looked at adjuvant pembrolizumab in patients with stage III melanoma.

The combination of nivolumab (Opdivo) and ipilimumab (Yervoy) showed a sustained survival benefit compared with nivolumab or ipilimumab alone, according to 5-year follow-up results.
Gaudenz Danuser, PhD, discusses how the activation of RacP29S impacts the treatment of patients with melanoma. His lab has been studying the Rac molecule for around 20 years. The RacP29S mutation most commonly appears in melanoma, but it has since been discovered in a few other cancer types as well.

Combining a BRAF inhibitor with a MEK inhibitor causes endoplasmic reticulum stress in BRAF wild-type, NRAS­-mutated melanoma cells, resulting in significant antitumor activity.
Allison Betof Warner, MD, PhD, discusses the excitement surrounding the findings from the COMBI-i trial that was presented at the 2019 ASCO Annual Meeting. This trial investigated the combination of PD-1 inhibitor spartalizumab with dabrafenib plus trametinib in patients with advanced BRAF V6000mutant melanoma.

Alexander N. Shoushtari, MD, discusses the significance of the findings from the first-in-human trial evaluating tebentafusp, a TCR–CD3 bispecific, in patients with advanced melanoma.

The combination of nivolumab and ipilimumab did not show a statistically significant improvement in recurrence-free survival compared with single-agent nivolumab as an adjuvant treatment for patients who have had complete surgical removal of stage IIIB/C/D or stage IV melanoma and whose tumors expressed PD-L1

The combination of vemurafenib and cobimetinib led to a 5-year overall survival rate of nearly 40% in patients with BRAF V600E–mutant metastatic melanoma who had not received prior therapy with a BRAF inhibitor.
Georgina V. Long, BSc, PhD, MBBS, FRACP, discusses the rationale for the combination of nivolumab (Opdivo) and ipilimumab (Yervoy) in patients with melanoma who have brain metastases.
David Polsky, MD, PhD, discusses potential applications of circulating tumor DNA as a biomarker in melanoma.

Jason J. Luke, MD, FACP, discusses the treatment landscape of metastatic melanoma.
Adil Daud, MD, clinical professor, Department of Medicine (Hematology/Oncology) and director, Melanoma Clinical Research, University of California, San Francisco Helen Diller Family Comprehensive Cancer Center, discusses research evaluating interferon gamma as a potential biomarker of response in melanoma.

Quickly following on the approval of single agents, adjuvant immunotherapy combinations are quickly progressing through development, with promising signs of clinical activity seen in phase II studies, according to a presentation by Jeffrey S. Weber, MD, PhD, at the 37th Annual CFS®.

Jeffrey S. Weber, MD, PhD, discusses biomarker research in the CheckMate-238 trial looking at patients with resected stage III/IV melanoma.
Georgina V. Long, BSc, PhD, MBBS, FRACP, discusses how to efficiently enroll patients with melanoma who have brain metastases in clinical trials.
Jeffrey S. Weber, MD, PhD, discusses the future of checkpoint inhibitors in melanoma.

The European Commission has approved nivolumab at a flat dosing schedule of either 240 mg over 30 minutes every 2 weeks, or 480 mg infused over 60 minutes every 4 weeks, for the adjuvant treatment of patients with melanoma who have involvement of lymph nodes or metastatic disease who have undergone complete resection.
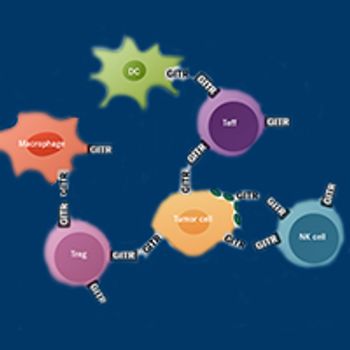
Immunotherapies designed to exploit the host immune system to specifically target cancer cells exploded onto the oncology scene in the mid-1980s, when the first such agents started to show success in melanoma and renal cell carcinoma.
Georgina V. Long, BSc, PhD, MBBS, FRACP, discusses the sequence of local therapy in patients with melanoma who have brain metastases.

Jeffrey S. Weber, MD, PhD, shares toxicity data from the randomized, double-blind, phase III CheckMate-238 trial in resected stage III or IV melanoma.