Ovarian Cancer
Latest News


Ubamatamab Monotherapy Elicits Responses in Heavily Pretreated Ovarian Cancer
Latest Videos

CME Content
More News

Treatment with mirvetuximab soravtansine elicited a statistically significant benefit vs investigator’s choice of chemotherapy for the OV28 abdominal/gastrointestinal symptom subscale in patients with folate receptor alpha–positive, advanced ovarian cancer.

González-Martín discusses the updated long-term efficacy and safety data from the PRIMA study, the importance of utilizing PARP inhibitors in patients with homologous recombination–deficient tumors, and the benefit observed with niraparib in those with homologous recombination–proficient tumors.

Antonio Gonzalez-Martin, MD, PhD, discusses new long-term progression-free survival data from the phase 3 PRIMA trial in advanced ovarian cancer.

Shannon N. Westin, MD, MPH, FACOG, explains the study design and outcomes of the SOLO-3 trial.

Dr Robert Coleman reviews data from the ARIEL-4 trial on rucaparib in recurrent ovarian cancer, sparking a discussion on PARP inhibitor use.

Robert Wenham, MD, MS, FACOG, FACS, discusses the importance of clinical trials in ovarian cancer.

Gavocabtagene autoleucel demonstrated positive topline results from the phase 1 portion of a phase 1/2 trial in patients with mesothelin-expressing solid tumors.

Pafolacianine used during ovarian cancer surgery identified additional tumors that were not detected with conventional means and not otherwise planned for resection, according to results from a phase 3 study recently published in the Journal of Clinical Oncology.

Manufacturers of 3 PARP inhibitors—niraparib, olaparib, and rucaparib—have voluntarily withdrawn indications for heavily pretreated patients with BRCA-mutated ovarian cancer due to safety concerns.

Olaparib plus bevacizumab was approved in China as a first-line maintenance treatment for homologous recombination deficiency–positive ovarian cancer following a response to platinum-based chemotherapy plus bevacizumab.

Maintenance treatment with niraparib produced a sustained and durable progression-free survival benefit in patients with primary advanced ovarian cancer who responded to first-line platinum-based chemotherapy, spanning biomarker subgroups.

Maurie Markman, MD, elaborates on the need to take a closer look at the benchmarks used in clinical trial designs in oncology.

A supplemental new drug application and a Type II variation has been submitted to the FDA and the European Medicines Agency, respectively, seeking the approval of rucaparib as frontline maintenance treatment in women with advanced ovarian cancer irrespective of biomarker status who have responded to first-line platinum-based chemotherapy.

The investigational carcinoembryonic antigen claudin 6 (CLDN6)–directed CAR T-cell therapy BNT211-01 displayed clinical activity both as monotherapy and in combination with a CLDN6-encoding mRNA vaccine in patients with CLDN6-positive relapsed/refractory advanced solid tumors.

Two years of olaparib maintenance therapy elicited a long-term overall survival benefit vs placebo in patients with newly diagnosed advanced ovarian cancer harboring a BRCA mutation.
Maintenance olaparib plus bevacizumab following first-line standard-of-care treatment improved overall survival in patients with newly diagnosed advanced ovarian cancer, particularly those with homologous recombination deficiency.

Pembrolizumab monotherapy and in combination with anlotinib demonstrated encouraging efficacy and safety when administered to patients with refractory or platinum-resistant recurrent high-grade serous ovarian cancer.
In this fifth episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, share key takeaways from their discussion on how to best individualize care for patients with ovarian cancer.

In this fourth episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss key developments made with PARP inhibitors in the ovarian cancer treatment paradigm.
In this third episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, explain the differences between germline and somatic testing in patients with ovarian cancer.
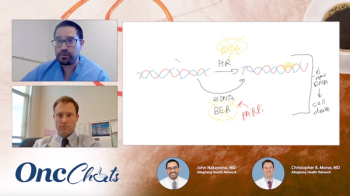
In this second episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss how to counsel patients with ovarian cancer whose tumors harbor BRCA mutations.
In this first episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss how BRCA mutational status affects treatment decisions for patients with ovarian cancer.

Maintenance therapy with niraparib significantly improved progression-free survival compared with placebo in Chinese patients with newly diagnosed advanced ovarian cancer who achieved a complete response or partial response to first-line chemotherapy.

The European Medicines Agency Committee for Medicinal Products for Human Use has recommended that rucaparib no longer be used as monotherapy for the third-line treatment of patients with platinum-sensitive, relapsed or progressive BRCA-mutated high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer.

The combination of ofranergene obadenovec and paclitaxel failed to elicit a statistically significant improvement in progression-free survival or overall survival compared with paclitaxel alone in patients with platinum-resistant ovarian cancer, missing the coprimary end points of the OVAL trial.