Immuno-Oncology
Latest News
Latest Videos

CME Content
More News

The oncolytic vaccine CG0070 plus pembrolizumab demonstrated encouraging response rates and a tolerable safety profile in patients with non–muscle invasive bladder cancer unresponsive to Bacillus Calmette-Guérin.

Nivolumab alone or in combination with ipilimumab produced encouraging responses that were maintained over time in patients with recurrent or metastatic cervical cancer.

In this sixth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, highlight the success seen with checkpoint inhibitors in gynecologic cancers, biomarkers of interest, and recent approvals in the space.

The combination of NT-I7 and pembrolizumab showed significant clinical activity in checkpoint inhibitor–naïve, relapsed/refractory microsatellite stable colorectal cancer and pancreatic cancer without liver metastasis.

The United Kingdom’s National Institute for Health and Care Excellence has issued final guidance recommending the use of nivolumab plus chemotherapy as a treatment option for patients with HER2-negative advanced stomach and esophageal cancer.

The combination of the p38 MAPK inhibitor ARRY-614 plus nivolumab with or without ipilimumab was well tolerated and elicited disease control in high-risk, PD-(L)1–refractory patients with advanced solid tumors.
PDS0101 given in conjunction with chemoradiation elicited an 100% overall response rate, along with tumor shrinkage greater than 60%, in 9 patients with high-risk, locally advanced cervical cancer, according to results of the phase 2 IMMUNOCERV trial.
In this fifth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, discuss PD-1 blockade, potential biomarkers of response to treatment with immunotherapy, and the potential for these agents in the neoadjuvant setting.
The combination of pembrolizumab and physician’s choice of chemotherapy produced a statistically significant overall survival benefit vs chemotherapy plus placebo in patients with HER2-negative, locally advanced, unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma.
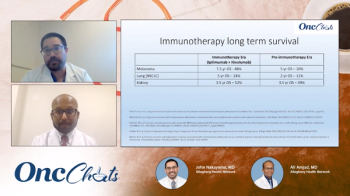
In this fourth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, highlight long-term survival data reported with immunotherapy approaches in patients with gynecologic cancers.
The Center for Drug Evaluation of the China National Medical Products Administration has granted a breakthrough therapy designation to ivonescimab plus docetaxel for patients with locally advanced or metastatic non–small cell lung cancer who failed to respond to a prior PD-(L)1 inhibitor plus platinum-based doublet chemotherapy.

The combination of sotigalimab and pembrolizumab showed encouraging antitumor activity and was well tolerated in the frontline setting of patients with metastatic melanoma.

In this third episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, review the 5 C's to consider when conducting research in gynecologic cancers and beyond.

The Chemotherapy Foundation Symposium® returns to New York City for its 40th annual meeting with a 3-day program that will deliver the latest updates across the gamut of oncology care.

Nabil F. Saba, MD, FACP, expanded on efficacy data from a phase 2 trial investigating pembrolizumab plus cabozantinib in patients with recurrent metastatic head and neck squamous cell carcinoma and current challenges and safety concerns in selecting patients for evaluation.

A doublet regimen comprised of pembrolizumab and cabozantinib elicited encouraging responses in patients with recurrent metastatic head and neck squamous cell carcinoma, meeting the primary end point of a phase 2 trial.

In this second episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, discuss the safety and efficacy of immunotherapy vs standard chemotherapy in patients with gynecologic cancers.

In this first episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, explain how immunotherapy has changed practice for the treatment of patients with gynecologic cancers.

Neil D. Gross, MD, FACS, discusses the current deficits in the treatment of patients with cutaneous squamous cell carcinoma in the curative setting and avenues for continued research generated by the primary analysis of the phase 2 trial examining neoadjuvant cemiplimab in this population.

Rana R. McKay, MD, discusses the evolution of frontline treatment options for metastatic RCC, and the key clinical trials that have shifted the treatment paradigms in metastatic castration-resistant prostate cancer, nonmetastatic castration-resistant prostate cancer, and advanced prostate cancer.

Adjuvant treatment with nivolumab resulted in a statistically significant and clinically meaningful improvement in recurrence-free survival over placebo in patients with completely resected stage IIB or IIC melanoma.
Pembrolizumab, alone and in combination with chemotherapy, maintained an overall survival benefit compared with cetuximab plus chemotherapy for patients with recurrent or metastatic head and neck squamous cell carcinoma at 4 years.
Sebastian C. Schmid, MD, discusses the results of the RACE IT trial, what the data mean for patients with locally advanced urothelial carcinoma, and the next steps for researching neoadjuvant radioimmunotherapy in this patient population.
Response to pembrolizumab monotherapy was observed regardless of prior response to chemotherapy in patients with advanced urothelial cancer.

The addition of dostarlimab to chemotherapy led to an improvement in objective response rate vs pembrolizumab plus chemotherapy in patients with newly diagnosed metastatic nonsquamous non–small cell lung cancer.